A theoretical study of gas adsorption on silicene nanoribbons and its application in a highly sensitive molecule sensor†
S. M. Aghaeia,
M. M. Monshia and
I. Calizo*ab
aQuantum Electronic Structures Technology Lab, Department of Electrical and Computer Engineering, Florida International University, Miami, Florida 33174, USA. E-mail: icalizo@fiu.edu
bDepartment of Mechanical and Materials Engineering, Florida International University, Miami, Florida 33174, USA
First published on 29th September 2016
Abstract
Inspired by recent successes in the development of two-dimensional based gas sensors capable of single gas molecule detection, we investigate the adsorption of gas molecules (N2, NO, NO2, NH3, CO, CO2, CH4, SO2, and H2S) on silicene nanoribbons (SiNRs) using density functional theory (DFT) and nonequilibrium Green's function (NEGF) methods. The most stable adsorption configurations, adsorption sites, adsorption energies, charge transfer, quantum conductance modulation, and electronic properties of gas molecules on SiNRs are studied. Our results indicate that NO, NO2, and SO2 are chemisorbed on SiNRs via strong covalent bonds, suggesting its potential application for disposable gas sensors. In addition, CO, NH3, and H2S are chemisorbed on SiNRs with moderate adsorption energy, alluding to its suitability as a highly sensitive gas sensor. The quantum conductance is detectably modulated by chemisorption of gas molecules which can be attributed to the charge transfer from the gas molecule to the SiNR. Other studied gases are physisorbed on SiNRs via van der Waals interactions. It is also found that the adsorption energies are enhanced by doping SiNRs with either B or N atoms. Our results suggest that SiNRs show promise in gas molecule sensing applications.
I Introduction
Although the global development of chemical activities in the past century is a consequence of human demands, it also affects human health and life quality from the associated environmental release of poisonous substances in the forms of solids, liquids and vapours. Therefore, gas detection is vital for managing chemical processes to prevent health hazards such as air pollution, device contamination, and for medical diagnosis. Today, in developing novel gas sensing materials with high sensitivity, even down to the single molecule level, high selectivity, high stability, quick response and recovery, and low power consumption are of considerable importance. Graphene, the first discovered two-dimensional (2D) material,1,2 renders outstanding properties such as high surface-volume ratio, low electronic temperature noise, remarkably high carrier mobility, high chemical and thermal stability, and fast response time, which make it promising in the development of ultrasensitive sensors with high packing density, higher sensitivity, better selectivity, faster recoverability, and lower power consumption.3–5 The potential application of graphene in gas sensors has been widely studied both experimentally6,7 and theoretically.8–10 However, the physisorption of common gas molecules such as CO2, CO, CH4, H2O, H2, N2, NO2, and NO on pristine graphene8 restrict its potential for single molecule detection.11,12 It is found that the sensing ability of graphene can be enhanced by introducing dopants or defects.9,10,13Inspired by the alluring properties of graphene, silicene, the silicon analogue of graphene, having a buckled honeycomb structure,14–16 has garnered considerable interest because of its remarkable properties including ferromagnetism,17 half-metallicity,18 quantum Hall effect,19 giant magnetoresistance,20 and superconductivity.21 Takeda and Shiraishi14 reported silicene for the first time in 1994 and Guzmán-Verri and Voon15 coined its name in 2007. Although free-standing silicene is not stable, it was experimentally fabricated on Ag,21–24 Ir,25 ZrB2,26 and ZrC.27 Using first-principles calculations, various substrates such as h-BN,28 SiC,29 GaS,30 graphene31 and ZnS32 which have weak van der Waals (vdW) interactions with silicene have been also studied to improve its stability. The extraordinary properties of silicene along with its compatibility with silicon based nanoelectronics may give the edge to silicene rather than graphene. Numerous potential applications of silicene in spintronics,33 field-effect transistors (FETs),34,35 and sensing devices36,37 have been proposed. Unlike a flat graphene sheet, the silicene honeycomb structure is buckled15,16 due to the tendency of silicon atoms to adopt sp3 and sp2 hybridization rather than only sp2 hybridization.22 This buckled structure makes it possible for the band gap of silicene to be tuned more intensively with an external electric field38 and with binding adsorbates39 as compared to graphene.40 Furthermore, silicene shows a considerably higher chemical reactivity for atoms41–46 and molecules47–53 adsorption than graphene due its buckled formation with a great deal of potential applications for silicene based nanoelectronic devices,54 hydrogen storage,45 thin film solar cell absorbers,46 hydrogen separation membranes,47 and molecule sensors.49–51,55
Similar to graphene, silicene is a zero band gap semimetal.15 It is expected that the effects of gas molecule adsorption on the electronic properties of a material without a band gap is much less than that of a semiconductor with an intrinsic band gap. A number of methods have been proposed to induce a band gap in a silicene sheet including doping,56 substrate effects,57 chemically functionalization,58,59 electric field,33 nanomesh and nanoholes.60–62 One of the possible methods to introduce a band gap in silicene is achieved by cutting the sheet into silicene nanoribbons (SiNRs).16,34,63 SiNRs have been grown on Ag (100), Ag (110), Au (110) substrates.64–67 Aufray et al. have synthesized an array of highly uniform parallel SiNRs with width of ∼1.7 nm on Ag (110) substrate.64 The intrinsic band gap and small dimension along with free reactive edges make nanoribbons more attractive than sheets for gas nanosensor applications. Several experimental and theoretical studies of graphene nanoribbons (GNRs) sensing properties have been already reported.68–71 The CO, NO, NO2, and O2 molecules are chemisorbed on armchair GNRs (AGNRs), while the adsorption of NH3 and CO2 on AGNRs is between weak chemisorption and strong physisorption.70 It has been found that CO, CO2, NO, NO2, and O2 molecules draw electrons from AGNRs, while NH3 donates its electrons into AGNRs. Little attention has been focused on molecule adsorption on SiNRs. A recent theoretical work by Osborn and Farajian has proven that ASiNRs can be used for detection of CO molecule due to its weak chemisorption on ASiNRs.72 They also found that H2O and O2 are strongly chemisorbed on ASiNRs, while CO2 and N2 barely affect ASiNR's conductance.
It remains an open question as to what is the adsorption behavior of nitrogen-, sulfur-, and other carbon-based gas molecules, including NO, NO2, NH3, SO2, H2S, and CH4 which are all of great practical interest for environmental, medical, and industrial applications, on SiNRs. In this paper, we employed first-principles methods based on density functional theory (DFT) to investigate the adsorption behavior of CO, CO2, CH4, N2, NO, NO2, NH3, SO2, and H2S molecules on SiNRs. Our results promulgate the promising future of SiNR in the development of ultrahigh sensitive sensor platforms.
II Computational methods
All the calculations are carried out based on first-principles DFT combined with nonequilibrium Green's function (NEGF) implemented in Atomistix ToolKit (ATK) package.73–75 The Generalized Gradient Approximation of Perdew–Burke–Ernzerhof (GGA-PBE) with a double-ζ polarized basis set is adopted to solve Kohn–Sham equations and to expand electronic density. The density mesh cut off is set to be 150 Rydberg. The Grimme vdW correction (DFT-D2)76 is also employed to describe long-range vdW interactions.77 In order to take into account the vdW interactions, an additional term (EvdW) is added to the DFT total energy (EDFT):| EDFT-D2 = EDFT + EvdW |
Besides vdW interactions, since our systems have two subsystems: the SiNR (A) and the gas molecule (B), so-called basis set superposition errors (BSSE) are expected due to the incompleteness of the Linear Combination of Atomic Orbitals (LCAO) basis set. In an isolated A system, only the basis orbitals in the A system describe it. While, A and B are coupled, the basis orbitals in the system B will also be used to describe system A, resulting in a larger available basis set for system A. Consequently, there will be an artificial interaction which decreases the total energy. To eradicate BSSE, a counterpoise (cp) correction should be added to the total energy.78 Therefore, the total energy of the system considering the long-range vdW interactions and artificial attractions between two subsystems is:
| Etotal = EDFT-D2 + Ecp |
To avoid the mirroring interactions, a vacuum space of 25 Å is considered in x and y directions in which the structures are not periodic. The electronic temperature is kept constant at 300 K. All the structures are completely relaxed, prior to the calculations, up until the force and stress are less than 0.01 eV Å−1 and 0.005 eV Å−3, respectively. 1 × 1 × 21 k-points in the Brillouin zone are sampled for geometry optimization and 1 × 1 × 121 k-points for total energy, electronic properties, charge transfer, and electron transport calculations. To investigate the charge transfer and transport properties, the gas sensing system is divided into three regions: two electrode regions (left and right) and a scattering region (the central region), as illustrated in Fig. 1. The transmission coefficient T(ε) can be obtained from the retarded Green's function G(ε) using
| T(ε) = G(ε)ΓL(ε)G†(ε)ΓR(ε) |
Here ΓL(R) is the broadening function of the left (right) electrode which defined as
| C(ε) = G0T(ε) |
III Results and discussions
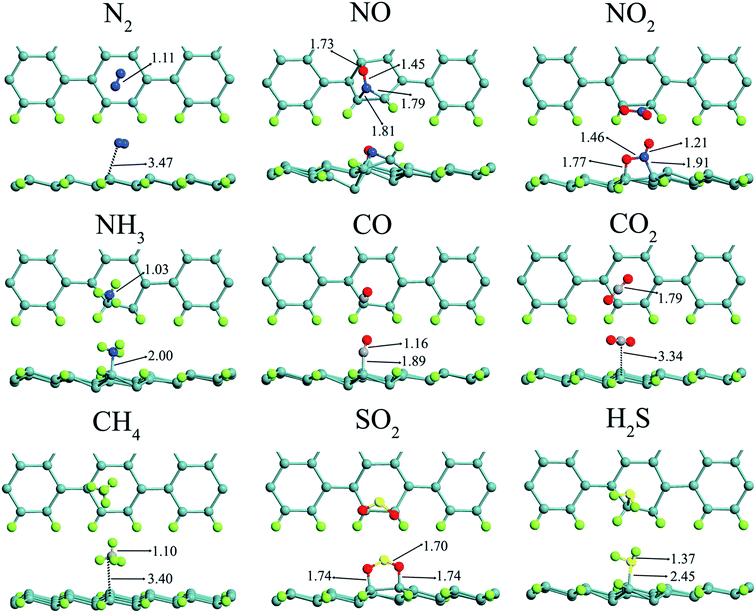
In this study, all the nanostructures are made of long and non-periodic 7-ASiNRs (L = 35 Å). Their edges are passivated by H while their surfaces are kept pristine because the surfaces are less reactive than the edges79 and silicene lacks complete sp3 hybridization.80 It has been reported that 7-ASiNR is a paramagnetic semiconductor with a band gap of ∼0.56 eV.62,64 The semiconducting ASiNR is chosen because it is expected that the adsorption of gas molecules has a much smaller effect on the electronic properties of zigzag SiNRs (ZSiNRs) which are metallic. To evaluate chemical sensing of SiNR-based nanosensors, single gas molecules (N2, NO, NO2, NH3, CO, CO2, CH4, SO2, and H2S) are initially placed about 3 Å from the ASiNRs surface. The adsorption behaviors of molecules on SiNR are investigated after full relaxation. The structural stability of the molecule's adsorption on SiNR is addressed using adsorption energy (Ead) which is| Ead = ESiNR+molecule − ESiNR − Emolecule |
As previously mentioned in Section II, the long range vdW interactions which come from non-local dispersion and artificial interactions are important factors in studying adsorption behaviour of gas molecules on SiNR. In order to examine the impact of vdW and counterpoise corrections on our calculations, the adsorption energy of gas molecules on SiNR and binding distances between gas molecules and SiNR are obtained by DFT-D2+cp (considering vdW dispersion and counterpoise correction) and PBE-GGA approximation and shown in Fig. 3. The results show that the absolute values of adsorption energy calculated by DFT-D2+cp are significantly more than that of GGA-PBE approximation. In addition, the optimum binding distances between molecules and SiNR obtained by DFT-D2+cp are a little less than calculated distances via GGA-PBE approximation.
The calculated adsorption energies and binding distances between the gases and ASiNR which are obtained by DFT-D2+cp method are also specified in Table 1. Our results show that CO is chemically adsorbed on SiNR with an adsorption energy of −0.92 eV. In the most stable structure, the CO molecule prefers to be adsorbed in a vertical orientation when the C atom is connected to a Si-edge atom with a covalent bond at a hill position with a binding distance of 1.89 Å. The bond length of the adsorbed CO is 1.16 Å which is little longer than that of an isolated CO molecule (1.14 Å). For CO2 and CH4, the results indicate that they are physisorbed on SiNR with a small adsorption energy of −0.31 and −0.18 eV, respectively. Both molecules prefer to be placed at hollow site after relaxation. The N2 molecule is energetically stable when it is horizontally situated at the center of a hexagonal silicon cell near the edge. The low adsorption energy of −0.31 eV shows that N2 is physically adsorbed on SiNRs. However, SiNR is highly reactive to other N-based gas molecules such as NH3, NO2, and NO, whose adsorption energies are −1.12, −2.53, −2.68 eV, respectively. NH3 is chemically adsorbed on top of Si-edge atom at a hill position with the formation of a N–Si covalent bond (2 Å). The adsorption energy of NO2 and NO is even less than −2 eV showing a very strong chemisorption. For the NO2 molecule, the N–O bond interacts with the Si–Si bond of the SiNR at the edge, where the N–Si and O–Si covalent bonds' length are 1.91 and 1.77 Å, respectively. The chemisorption of the NO molecule on SiNR is even stronger than that of NO2 molecule. The N atom is bonded to Si–Si atoms on the edge of the SiNR to form a N–Si–Si triangle with N–Si and Si–Si bond lengths of 1.81 and 2.32 Å, respectively. The O atom is also connected to another Si atom on top of the Si–hexagon with a bond length of 1.73 Å. The adsorption energy of the H2S molecule on top of the SiNR is −0.64 eV, showing that the adsorption of the molecule is a weak chemisorption. Strong chemisorption is also observed for the SO2 molecule, whose adsorption energy is −2.63 eV. In this case, the O atoms of SO2 molecule are bonded to Si–Si bond at the SiNR edge, where the covalent bond lengths are 1.70 Å.
| Device | Gas | Ead (eV) | D (Å) | ρ (e) | Device | Gas | Ead (eV) | D (Å) | ρ (e) | Device | Gas | Ead (eV) | D (Å) | ρ (e) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a From ref. 39, 48–51 and 81. | ||||||||||||||
| P-SiNR | N2 | −0.30 | 3.47 | −0.110 | P-SiNR | 2N2 | −0.57 | 3.36 | −0.097 | DB-SiNR | N2 | −0.32 | 3.28 | −0.090 |
| NO | −2.68 | 1.73 | −0.892 | 2NO | −5.32 | 1.72 | −0.888 | NO | −3.23 | 1.74 | −0.908 | |||
| NO2 | −2.53 | 1.77 | −0.851 | 2NO2 | −5.08 | 1.78 | −0.849 | NO2 | −2.94 | 1.92 | −0.848 | |||
| NH3 | −1.12 | 2.00 | −0.535 | 2NH3 | −2.18 | 2.00 | −0.493 | NH3 | −1.24 | 1.98 | −0.520 | |||
| CO | −0.92 | 1.89 | −0.406 | 2CO | −1.78 | 1.89 | −0.386 | CO | −1.52 | 1.83 | −0.395 | |||
| CO2 | −0.31 | 3.34 | −0.101 | 2CO2 | −0.58 | 3.38 | −0.093 | CO2 | −0.35 | 3.27 | −0.100 | |||
| CH4 | −0.18 | 3.40 | +0.025 | 2CH4 | −0.32 | 3.41 | +0.021 | CH4 | −0.22 | 3.33 | +0.020 | |||
| SO2 | −2.64 | 1.74 | +0.626 | 2SO2 | −5.11 | 1.73 | +0.620 | SO2 | −2.72 | 1.80 | −0.615 | |||
| H2S | −0.64 | 2.45 | −0.342 | 2H2S | −1.25 | 2.45 | −0.375 | H2S | −0.79 | 2.45 | −0.323 | |||
| N-SiNR | N2 | −0.35 | 2.59 | −0.151 | B-SiNR | N2 | −0.55 | 1.60 | −0.352 | P-Silicenea | N2 | −0.08 | — | +0.005 |
| NO | −3.74 | 1.72 | +0.138 | NO | −3.82 | 1.38 | −0.233 | NO | −0.57 | 1.99 | +0.46 | |||
| NO2 | −3.60 | 1.75 | −0.041 | NO2 | −3.53 | 1.55 | −0.162 | NO2 | −1.53 | 1.75 | +0.82 | |||
| NH3 | −1.35 | 1.98 | −0.559 | NH3 | −1.14 | 1.63 | −0.663 | NH3 | −0.60 | 2.04 | +0.10 | |||
| CO | −1.89 | 2.00 | −0.274 | CO | −1.59 | 1.50 | −0.452 | CO | −0.10 | 3.24 | +0.03 | |||
| CO2 | −0.35 | 2.70 | −0.140 | CO2 | −0.32 | 3.28 | −0.110 | CO2 | −0.04 | — | ∼0.00 | |||
| CH4 | −0.27 | 3.57 | +0.016 | CH4 | −0.25 | 3.39 | +0.014 | CH4 | −0.08 | — | +0.004 | |||
| SO2 | −3.48 | 1.75 | −0.210 | SO2 | −2.95 | 1.57 | −0.204 | SO2 | −1.08 | 2.17 | +0.17 | |||
| H2S | −1.86 | 2.40 | −0.305 | H2S | −0.90 | 1.98 | −0.407 | H2S | −0.212 | 2.28 | — | |||
To sum up, the interaction of N2, CO2, and CH4 gas molecules with a SiNR is mostly vdW type adsorbing via physisorption. A SiNR cannot be an appropriate sensor for detection of NO, NO2, and SO2 gas molecules because of their strong chemisorption on silicene. However, SiNRs can be considered as a disposable molecule sensor for detection of NO, NO2, and SO2. Besides, the strong covalent bonding of these molecules to SiNR makes it possible to tune the electronic properties of SiNR for nanoelectronics applications, as will be discussed later. Finally, the moderate adsorption energies of NH3, CO, and H2S gas molecules make SiNRs a promising material for sensitive gas sensors or gas filters because they can be easily desorbed from the SiNR by heating. It should also be noted that environmental gas molecules (N2, CO2, O2, and H2O) can change the NH3 and CO sensing capability of SiNRs. Unlike physisorption of N2 and CO2 gas molecules on SiNR, the water and oxygen molecules are strongly chemisorbed on SiNR.72 Therefore, it is critical that water and oxygen molecules be removed from the environment to preserve the proper sensing capability of SiNRs.
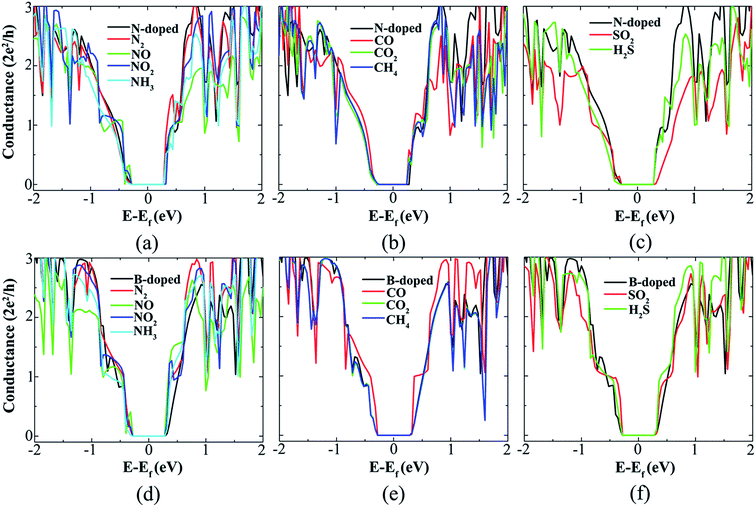
To investigate the effects of gas molecules on the conductance of SiNR, the quantum conductances of the pristine SiNR before and after adsorption are calculated, as shown in Fig. 4. Upon adsorption of N-based gas molecules, the band gap of pristine SiNR (0.56 eV) is decreased slightly for N2 (0.52 eV) and NH3 (0.48 eV) and is somewhat increased for NO2 (0.68 eV) and NO (0.68 eV). The conductances of SiNR before and after adsorption of N2 molecule are similar due to the physisorption of N2 gas molecule on SiNR, showing insensitivity of SiNR sensor to N2 gas molecule. However, the overall conductances of SiNRs are detectably dropped after NH3, NO2, and NO, confirming their chemisorption on SiNRs. The conductance reduction for a NO molecule is more vivid than other N-based gas molecules which are consistent with the calculated adsorption energies. For C-based gas molecules, the band gap of pristine SiNR is preserved for CO2 (0.56 eV) and slightly decreased for CH4 (0.52 eV) and CO (0.48 eV). The overall conductance of pristine SiNR is visibly reduced after CO adsorption, while barely changed after CO2 adsorption, and almost unchanged after CH4 adsorption which confirms the calculated adsorption energies for C-based gas molecules where the CO adsorption energy is more negative than others. Upon adsorption of S-based gas molecules, the band gap of pristine SiNR is unaltered for SO2 (0.56 eV) and decreased for H2S (0.48 eV) gas molecule. A reduction in the conductance of the SiNR from SO2 and H2S adsorption is also observed, confirming the sensing capability of SiNR for SO2 and H2S gas molecules.
 | ||
| Fig. 4 Quantum conductance of pristine SiNR before and after (a) N2, NO, NO2, NH3, (b) CO, CO2, CH4, (c) SO2 and H2S gas molecules adsorption. | ||
The electron charge transfer between gas molecules and SiNR is also investigated using Mulliken population analysis, as shown in Table 1. The positive value of charge indicates a charge transfer from SiNR surface to molecule. N2, NO, NO2, NH3, CO, CO2, and H2S are electron-donating gas molecules, while CH4 and SO2 have an electron-withdrawing capability. A large amount of charge transfer is observed for NO (−0.892 e), NO2 (−0.851 e), NH3 (−0.535 e) and CO (−0.406 e) due to their strong electron-donating characteristics. These large charge transfer molecules to SiNR are also correlated to the strong binding energies of the gas molecules chemisorption on SiNR. The calculated adsorption energies of all studied gas molecules on SiNR are distinctly larger than those of GNR,70 showing higher sensitivity of SiNR toward the gas molecules. Furthermore, comparing these findings with gas molecules adsorption on pristine silicene,39,48–50,81 see Table 1, the value of adsorption energy and charge transfer are much larger in favor of SiNR, pointing to the great potential of SiNRs for application as a highly sensitive molecule sensor.
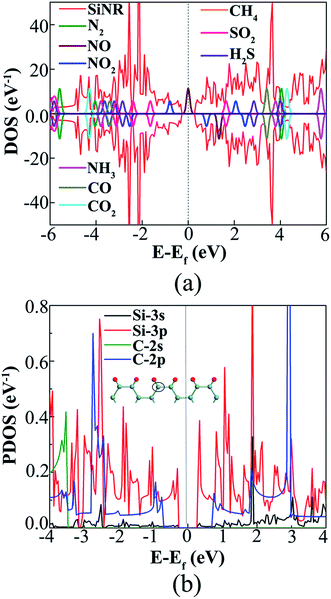
The origin of the chemisorption of NO, NO2, NH3, CO, SO2 and H2S and physisorption of N2, CO2, and CH4 gas molecules on SiNR can be revealed by studying their density of states (DOS). Fig. 5(a) presents the total DOS of pristine SiNR and the gas molecules. The frontier molecular orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), of NO, NO2, and SO2 are closer to the Fermi level of SiNR than that of NH3, CO, and H2S, representing that SiNRs show higher sensitivity for the former molecules. Furthermore, these molecules have higher reactivity to SiNRs as compared to N2, CO2, and CH4 which are physisorbed on the SiNR. These results show reasonable agreement with the calculated adsorption energies and are consistent with earlier research on gas molecule adsorption on silicene sheets.48–51 As previously mentioned, silicene is more reactive than graphene because of its tendency to adopt sp2 + sp3 hybridization over sp2 hybridization.22 The difference between the reactivity of silicene and graphene can be understood from Fig. 5(b), where PDOS of silicon/carbon atom located at the edge of SiNR/GNR is calculated. While both silicon (Ne 3s23p2) and carbon (1s22s22p2) contribute four valence electrons in the ribbon, the contributions of s orbitals are negligible near the Fermi level. Therefore, only p orbitals could be considered. Comparing the p orbital contributions of Si and C atoms near the Fermi level, it is clear that the corresponding electronic edge states of 7-ASiNR are closer than that of 7-AGNR to the Fermi level, causing stronger reactivity of SiNR for molecule adsorption.
To gain more insight into the chemisorption and physisorption of gas molecules, their electronic total charge densities are calculated, as depicted in Fig. 6. It can be seen that there is no electron orbital overlap between N2, CO2, and CH4 gas molecules and SiNR, confirming this fact that only weak physisorption takes place in these cases. In contrast, a strong orbital overlap is observed between NO, NO2, and SO2 gas molecules and SiNR, resulting in more orbital mixing and a larger charge transfer. It is clear that this strong orbital overlap results in significant changes to the electronic properties of SiNR. Finally, a moderate orbital overlap between NH3, CO, and H2S gas molecules and SiNR suggest the potential application of SiNR as a reusable gas sensor because the electronic properties of SiNR which are moderately changed can be retrieved by desorbing gas molecules from SiNR. The results correspond with calculated adsorption energies.
 | ||
| Fig. 6 Electronic total charge densities for the adsorption of N2, NO, NO2, NH3, CO, CO2, CH4, SO2, and H2S gas molecules on pristine ASiNR. | ||
In order to find the impact of nanoribbon width on the adsorption energy, we also considered the CO molecule adsorption on 5-ASiNR, 6-ASiNR, and 8-ASiNR. The values of adsorption energy of CO molecule on 5-ASiNR, 6-ASiNR, 7-ASiNR, and 8-ASiNR are −0.98, −0.95, −0.93, and −0.91 eV, respectively. This overall small decrease in absolute value of adsorption energy with decreasing surface coverage of the SiNR is in agreement with those previously reported for CO molecule adsorbed on AGNR.82
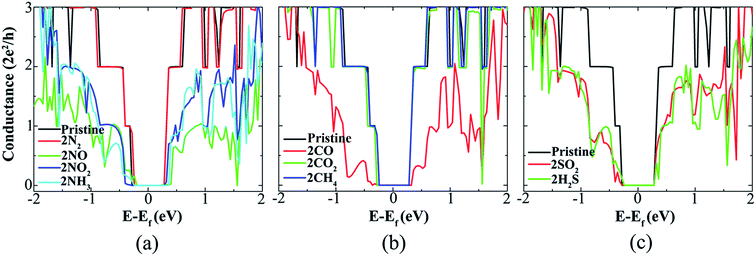
Since upon adsorption of individual gas molecules a conduction reduction is observed in the properties of SiNRs, significant alterations in the electronic properties of SiNRs are expected at higher concentrations of gas molecules. To evaluate this premise, a higher surface coverage with two gas molecules is considered and the impacts of gas molecules adsorption on the conduction of SiNR are investigated. Fig. 7 presents the most stable configurations of two adsorbed gas molecules on opposite edges of the SiNR. Based on the calculations, an individual gas molecule can be adsorbed anywhere at the SiNR's edges and its position will not change the quantum conductance. The second gas molecule can also be adsorbed on the same side or the opposite side of the first gas molecule. The results show that the conductance changes are almost the same for two cases, in agreement with the findings of Osborn et al.72 By calculating adsorption energies, it is found that adding an additional gas molecule to the system doubles the value of adsorption energies of gas molecules on the SiNR, see Table 1. It is clear that the NO, NO2, NH3, CO, SO2, and H2S sensing capability of SiNR, which is exposed by two gas molecules, is increased due to the significant reduction in the conductance of pristine SiNR and the strong chemisorption of gas molecules to the SiNR (their adsorption energies are less than −1 eV), as shown in Fig. 8. By performing Mulliken population analysis of the SiNR with one adsorbed molecule and comparing to the SiNR with two adsorbed molecules, it is found that there are no appreciable differences between the amounts of charge transfer, see Table 1.
 | ||
| Fig. 7 The most stable adsorption configurations for two N2, NO, NO2, NH3, CO, CO2, CH4, SO2, and H2S on pristine ASiNR. | ||
 | ||
| Fig. 8 Quantum conductance of pristine SiNR before and after (a) two-N2, -NO, -NO2, -NH3, (b) -CO, -CO2, -CH4, (c) -SO2 and -H2S gas molecules adsorption. | ||
It is predicted that the edges of SiNRs are not well controlled similar to GNRs.83,84 As a result, it seems difficult to achieve a fully edge hydrogenated SiNR without any dangling bond (DB) defects. It is found that edge DB defects are much more probable than surface DB defects in silicon nanowires.85–88 Therefore, it is necessary to investigate the sensing capability of a SiNR with an edge DB defect. To this end, a hydrogen atom is removed from one silicone edge atom, and only the gas adsorption above the DB defect is tested. The most stable adsorption configurations of gas molecules on SiNR with an edge DB defect are illustrated in Fig. 9.
Fig. 10 shows the conductance modulation of ASiNR with an edge DB defect upon adsorption of gas molecules. As one can see the edge DB defects do not limit the sensing capability of the SiNR. The absolute value of adsorption energies of all gas molecules on SiNR with an edge DB defect, see Table 1, are increased, leading to detectable modifications in SiNR's conductance. Based on their adsorption energies, it can be understood that NO (−3.23 eV), NO2 (−2.94 eV), NH3 (−1.24 eV), CO (−1.52 eV), and SO2 (−2.72 eV) are strongly chemisorbed on the SiNR with a DB defect, while H2S (−0.79 eV) is weakly chemisorbed on the SiNR. However, the N2 (−0.32 eV) and CH4 (−0.22 eV) molecules are still physisorbed on the SiNR with small adsorption energy. The Mulliken population analysis of the SiNR with an edge DB defect and adsorbed molecule shows that a DB defect slightly decreases the charge transfer between SiNR and gas molecules, see Table 1.
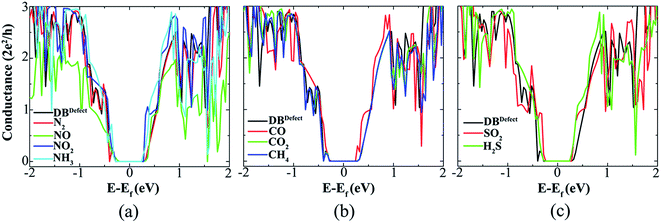 | ||
| Fig. 10 Quantum conductance of SiNR with an edge DB defect before and after (a) N2, NO, NO2, NH3, (b) CO, CO2, CH4, (c) SO2 and H2S gas molecules adsorption. | ||
Here, we study the effects of doping on the gas molecules sensing capability of SiNR. To this end, doping effects of SiNRs with N and B impurities have been considered. It has been found that a single N or B dopant prefers to be substituted with Si edge atoms because the formation energy of N or B impurity at the edge is lower than other positions of the nanoribbon.59 To focus on the effects of doping on the sensing capability of the SiNR, the gas molecules adsorption above the B and N impurities and their nearest neighbours are studied. It is discovered that the gas molecules energetically tend to be chemisorbed on B atom in B-doped SiNR, and chemisorbed on the Si atoms nearest to the N atom in N-doped SiNR. The adsorption energies of gas molecules on the doped SiNR are larger than that of pristine SiNR, proving that doping improves sensing capabilities of SiNR, see Table 1. These findings concur with previous studies on gas detection based on doped silicene.50
The most stable configuration of adsorbed gas molecules on N-doped and B-doped ASiNR are depicted in Fig. 11. For CO gas molecule on N-doped SiNR, two covalent bonds are formed between C and the two nearest Si atoms to the N atom, while, for B-doped SiNR, C atom and B atom are covalently bonded. The adsorption energies of CO molecule on N- and B-doped SiNR are 2.04 and 1.72, greater than that of pristine SiNR. The NO gas molecule is also strongly chemisorbed on N- and B-doped SiNR. Although three covalent bonds are formed between NO and nearest Si atoms to the N atom in N-doped SiNR, the adsorption of NO on B-doped SiNR totally destroys the configuration of atoms on the edge. The most stable configurations for NO2, NH3, and SO2 gas molecules adsorption on N- and B-doped SiNR are quite similar to the pristine SiNR. The only difference is that they prefer to be adsorbed on top of a B atom and the nearest Si atom to the N atom. The CO2 and CH4 molecules on the N- and B-doped SiNR and N2 on the N-doped SiNR are still physisorbed. However, the N2 molecule is chemically adsorbed on B-doped SiNR with adsorption energy of −0.55 eV which is 1.79 times greater than that of pristine SiNR. Similar to gas adsorption on pristine SiNR, the conductances of N-doped and B-doped SiNR are detectably changed upon adsorption of NO, NO2, NH3, CO, SO2, and H2S, see Fig. 12. Furthermore, doping a B atom into the SiNR can improve the N2 gas sensing capability of SiNR, compared to that of pristine SiNR. Charge transfer analysis shows that N2, NO2, NH3, CO, CO2, H2S, and SO2 act as donors, while NO and CH4 act as acceptors for N-doped SiNR. However, for B-doped SiNR, all gases have electron donating capability except CH4, see Table 1.
Finally, a design of SiNR-based sensor to detect gas molecules is proposed, as shown in the inset of Fig. 13(a). Two semi-infinite 4-ZSiNRs as the leads and a 98 Å long 7-ASiNR gas sensing zone are the main elements of the device. We have assumed that 5 gas molecules with an average distance of 18 Å are adsorbed on the edge sites of the detection zone. By applying a bias voltage across the leads, conductance can be measured before and after adsorption. To assess the sensing performance of the proposed device for the gas molecules the current versus bias voltage is calculated in Fig. 13(a). For clarity and brevity, the I–Vbias curves for 7-ASiNR before and after adsorption of NO2 and NO molecules are considered because the currents induced by other molecules are much smaller than that of NO2 and NO molecules. As can be seen, the current is always almost zero for pristine 7-ASiNR before adsorption of molecules since 7-ASiNR is a semiconductor with a direct band gap of 0.56 eV, as shown in Fig. 13(b). However, upon NO2 adsorption, the current remarkably increases as a function of increasing voltage bias, the curve is almost linear indicating a metallic behavior. The current can be also increased by NO adsorption; however, the current value is much smaller than that of NO2. These results can be confirmed by analyzing the band structures of 7-ASiNR after NO and NO2 adsorption, as shown in Fig. 13(b). As mentioned before, the gas molecules are able to tune the electronic properties of SiNRs. The adsorption of NO2 and NO on 7-ASiNR can transform the system to n-type and p-type semiconductors, respectively, due to the deep defect states induced by gas molecules, as shown in Fig. 13(b). These n- and p-type semiconducting SiNRs can be applied in nanoscale electronic devices such as a p–n junction diode and n- or p-type FETs. It is also expected that NO2 gas molecule adsorption by SiNR can give rise to a conductance enhancement because of the metallic behavior of SiNR after NO2 molecule adsorption. However, for NO molecule adsorption, the conductance may be enhanced by some amount since the NO adsorption makes the SiNR a p-type semiconductor.
IV Conclusions
In summary, we employed first-principle calculations to explore the adsorption geometry, adsorption energy, charge transfer, electronic band structure, and quantum conductance modulation of SiNRs with gas molecules (N2, NO, NO2, NH3, CO, CO2, CH4, SO2, and H2S) adsorption. Our results reveal that SiNRs are capable of detecting CO, NH3, and H2S with high sensitivity because they are chemically adsorbed on the SiNR and transfer a few electrons to the SiNR. Quantum conductance modulation of SiNRs is clearly detectable upon chemisorption of gas molecules. Furthermore, SiNRs are not appropriate to serve as a sensor for NO, NO2, and SO2 due to the fact that they are strongly chemisorbed with covalent bonds to SiNRs and transfer a large amount of electrons to the SiNR. However, the strong bonding of NO and NO2 molecules is an effective way to tune the electronic properties of SiNRs to fabricate p-type and n-type transistors, respectively. The other gas molecules are physisorbed. We also found that increasing the density of gas molecules will result in more significant changes in quantum conductance. In addition, dangling bond defects which are unavoidable through fabrication process will not hinder the sensing capability of SiNRs. The sensing capability of a SiNR-based sensor can also be increased by either N or B doping. It is found that doping a SiNR with a B atom can enhance the detection capability of N2 gas molecules. On the basis of our results, SiNRs can be considered as a promising material to detect individual gas molecules.Acknowledgements
This work was supported in part by the Florida Education Fund's McKnight Junior Faculty Fellowship.References
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed.
- S. Basu and P. Bhattacharyya, Sens. Actuators, B, 2012, 173, 1–21 CrossRef CAS.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- Q. He, S. Wu, Z. Yin and H. Zhang, Chem. Sci., 2012, 3, 1764–1772 RSC.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301–306 CrossRef CAS PubMed.
- S. Some, Y. Xu, Y. Kim, Y. Yoon, H. Qin, A. Kulkarni, T. Kim and H. Lee, Sci. Rep., 2013, 3, 1868 Search PubMed.
- O. Leenaerts, B. Partoens and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 125416 CrossRef.
- Y. Zhang, Y. Chen, K. Zhou, C. Liu, J. Zeng, H. Zhang and Y. Peng, Nanotechnology, 2009, 20, 185504 CrossRef PubMed.
- J. Dai, J. Yuan and P. Giannozzi, Appl. Phys. Lett., 2009, 95, 232105 CrossRef.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Q. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137 CrossRef CAS PubMed.
- G. Lu, L. E. Ocola and J. Chen, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- J. Dai and J. Yuan, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 165414 CrossRef.
- K. Takeda and K. Shiraishi, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 14916 CrossRef CAS.
- G. G. Guzmán-Verri and L. L. Y. Voon, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 075131 CrossRef.
- S. Cahangirov, M. Topsakal, E. Aktürk, H. Şahin and S. Ciraci, Phys. Rev. Lett., 2009, 102, 236804 CrossRef CAS PubMed.
- X. Q. Wang, H. D. Li and J. T. Wang, Phys. Chem. Chem. Phys., 2012, 14, 3031–3036 RSC.
- F. Zheng and C. Zhang, Nanoscale Res. Lett., 2012, 7, 422 CrossRef PubMed.
- C. C. Liu, W. X. Feng and Y. G. Yao, Phys. Rev. Lett., 2011, 107, 076802 CrossRef PubMed.
- C. Xu, G. Luo, Q. Liu, J. Zheng, Z. Zhang, S. Nagase, Z. Gao and J. Lu, Nanoscale, 2012, 4, 3111–3317 RSC.
- L. Chen, B. Feng and K. Wu, Appl. Phys. Lett., 2013, 102, 081602 CrossRef.
- P. Vogt, P. De Padova, C. Quaresima, J. Avila, E. Frantzeskakis, M. C. Asensio, A. Resta, B. Ealet and G. Le Lay, Phys. Rev. Lett., 2012, 108, 155501 CrossRef PubMed.
- B. Feng, Z. Ding, S. Meng, Y. Yao, X. He, P. Cheng, L. Chen and K. Wu, Nano Lett., 2012, 12, 3507–3511 CrossRef CAS PubMed.
- J. Mannix, B. Kiraly, B. L. Fisher, M. C. Hersam and N. P. Guisinger, ACS Nano, 2014, 8, 7538–7547 CrossRef PubMed.
- L. Meng, Y. Wang, L. Zhang, S. Du, R. Wu, L. Li, Y. Zhang, G. Li, H. Zhou and W. A. Hofer, et al., Nano Lett., 2013, 13, 685–690 CrossRef CAS PubMed.
- A. Fleurence, R. Friedlein, T. Ozaki, H. Kawai, Y. Wang and Y. Yamada- Takamura, Phys. Rev. Lett., 2012, 108, 245501 CrossRef PubMed.
- T. Aizawa, S. Suehara and S. Otani, J. Phys. Chem. C, 2014, 118, 23049–23057 CAS.
- T. P. Kaloni, M. Tahir and U. Schwingenschlögl, Sci. Rep., 2013, 3, 3192 CAS.
- H. Liu, J. Gao and J. Zhao, J. Phys. Chem. C, 2013, 117, 10353 CAS.
- Y. Ding and Y. Wang, Appl. Phys. Lett., 2013, 103, 043114 CrossRef.
- Y. Cai, C.-P. Chuu, C. M. Wei and M. Y. Chou, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 245408 CrossRef.
- M. Houssa, B. van den Broek, E. Scalise, G. Pourtois, V. V. Afanas'eva and A. Stesmansa, Phys. Chem. Chem. Phys., 2013, 15, 3702 RSC.
- W.-F. Tsai, C.-Y. Huang, T.-R. Chang, H. Lin, H.-T. Jeng and A. Bansil, Nat. Commun., 2013, 4, 1500 CrossRef PubMed.
- Z. Ni, Q. Liu, K. Tang, J. Zheng, J. Zhou, R. Qin, Z. Gao, D. Yu and J. Lu, Nano Lett., 2012, 12, 113–118 CrossRef CAS PubMed.
- L. Tao, E. Cinquanta, D. Chiappe, C. Grazianetti, M. Fanciulli, M. Dubey, A. Molle and D. Akinwande, Nat. Nanotechnol., 2015, 10, 227–231 CrossRef CAS PubMed.
- H. Sadeghi, S. Bailey and C. J. Lambert, Appl. Phys. Lett., 2014, 104, 103104 CrossRef.
- R. G. Amorim and R. H. Scheicher, Nanotechnology, 2015, 26, 154002 CrossRef PubMed.
- N. D. Drummond, V. Zólyomi and V. I. Fal'ko, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 075423 CrossRef.
- T. P. Kaloni, G. Schreckenbach and M. S. Freund, J. Phys. Chem. C, 2014, 118, 23361–23367 CAS.
- P. Lazar, F. Karlický, P. Jurečka, M. Kocman, E. Otyepková, K. Šafářová and M. Otyepka, J. Am. Chem. Soc., 2013, 135, 6372–6377 CrossRef CAS PubMed.
- X. Lin and J. Ni, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 075440 CrossRef.
- J. Sivek, H. Sahin, B. Partoens and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 085444 CrossRef.
- H. Sahin and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 085423 CrossRef.
- G. A. Tritsaris, E. Kaxiras, S. Meng and E. Wang, Nano Lett., 2013, 13, 2258–2263 CrossRef CAS PubMed.
- J. Wang, J. Li, S.-S. Li and Y. Liu, J. Appl. Phys., 2013, 114, 124309 CrossRef.
- B. Huang, H. J. Xiang and S.-H. Wei, Phys. Rev. Lett., 2013, 111, 145502 CrossRef PubMed.
- W. Hu, X. Wu, Z. Li and J. Yang, Phys. Chem. Chem. Phys., 2013, 15, 5753 RSC.
- J.-W. Feng, Y.-J. Liu, H.-X. Wang, J.-X. Zhao, Q.-H. Cai and X.-Z. Wang, Comput. Mater. Sci., 2014, 87, 218 CrossRef CAS.
- W. Hu, N. Xia, X. Wu, Z. Li and J. Yang, Phys. Chem. Chem. Phys., 2014, 16, 6957 RSC.
- J. Prasongkit, R. G. Amorim, S. Chakraborty, R. Ahuja, R. H. Scheicher and V. Amornkitbamrung, J. Phys. Chem. C, 2015, 119, 16934–16940 CAS.
- R. Chandiramoulia, A. Srivastavab and V. Nagarajan, Appl. Surf. Sci., 2015, 351, 662–672 CrossRef.
- G. García, M. Atilhan and S. Aparicio, Phys. Chem. Chem. Phys., 2015, 17, 16315–16326 RSC.
- X. Wang, H. Liu and S. T. Tu, RSC Adv., 2015, 5, 65255–65263 RSC.
- A. Kara, H. Enriquez, A. P. Seitsonen, L. C. Lew Yan Voon, S. Vizzini, B. Aufray and H. Oughaddou, Surf. Sci. Rep., 2012, 67, 1–18 CrossRef CAS.
- E. Akbari, Z. Buntat, A. Afroozeh, S. E. Pourmand, Y. Farhang and P. Sanati, RSC Adv., 2016, 6, 81647–81653 RSC.
- A. Lopez-Bezanilla, J. Phys. Chem. C, 2014, 118, 18788–18792 CAS.
- N. Gao, J. Li and Q. Jiang, Chem. Phys. Lett., 2014, 592, 222–226 CrossRef CAS.
- N. Gao, W. T. Zheng and Q. Jiang, Phys. Chem. Chem. Phys., 2012, 14, 257–261 RSC.
- S. M. Aghaei, M. M. Monshi, I. Torres and I. Calizo, RSC Adv., 2016, 6, 17046 RSC.
- F. Pan, Y. Wang, K. Jiang, Z. Ni, J. Ma, J. Zheng, R. Quhe, J. Shi, J. Yang, C. Chen and J. Lu, Sci. Rep., 2015, 5, 9075 CrossRef CAS PubMed.
- S. M. Aghaei and I. Calizo, J. Appl. Phys., 2015, 118, 104304 CrossRef.
- S. M. Aghaei and I. Calizo, in Proceeding of IEEE SoutheastCon (SECon-2015), Fort Lauderdale, April 9–12, 2015, pp. 1–6 Search PubMed.
- Y. Ding and J. Ni, Appl. Phys. Lett., 2009, 95, 083115 CrossRef.
- B. Aufray, A. Kara, S. Vizzini, H. Oughaddou, C. Leandri, B. Ealet and G. Le Lay, Appl. Phys. Lett., 2010, 96, 183102 CrossRef.
- P. De Padova, C. Quaresima, C. Ottaviani, P. M. Sheverdyaeva, P. Moras, C. Carbone, D. Topwal, B. Olivieri, A. Kara, H. Oughaddou, B. Aufray and G. Le Lay, Appl. Phys. Lett., 2010, 96, 261905 CrossRef.
- P. De Padova, O. Kubo, B. Olivieri, C. Quaresima, T. Nakayama, M. Aono and G. Le Lay, Nano Lett., 2012, 12, 5500–5503 CrossRef CAS PubMed.
- M. R. Tchalala, H. Enriquez, A. J. Mayne, A. Kara, S. Roth, M. G. Silly, A. Bendounan, F. Sirotti, T. Greber, B. Aufray, G. Dujardin, M. A. Ali and H. Oughaddou, Appl. Phys. Lett., 2013, 102, 083107 CrossRef.
- A. N. Abbas, G. Liu, B. Liu, L. Zhang, H. Liu, D. Ohlberg, W. Wu and C. Zhou, ACS Nano, 2014, 8, 1538–1546 CrossRef CAS PubMed.
- A. N. Abbas, G. Liu, A. Narita, M. Orosco, X. Feng, K. Müllen and C. Zhou, J. Am. Chem. Soc., 2014, 136, 7555 CrossRef CAS PubMed.
- B. Huang, Z. Y. Li, Z. R. Liu, G. Zhou, S. G. Hao, J. Wu, B. L. Gu and W. H. Duan, J. Phys. Chem. C, 2008, 112, 13442–13446 CAS.
- A. Saffarzadeh, J. Appl. Phys., 2010, 107, 114309 CrossRef.
- T. H. Osborn and A. A. Farajian, Nano Res., 2014, 7, 945–952 CrossRef CAS.
- J. Taylor, H. Guo and J. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245407 CrossRef.
- M. Brandbyge, J.-L. Mozos, P. Ordejon, J. Taylor and K. Stokbro, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 165401 CrossRef.
- Atomistix Toolkit version 2015.0, QuantumWise, Copenhagen, Denmark, see http://www.quantumwise.com Search PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787 CrossRef CAS PubMed.
- S. Grimme, C. Mück-Lichtenfeld and J. Antony, J. Phys. Chem., 2007, 111, 11199–11207 CAS.
- F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553 CrossRef.
- M. E. Dávila, A. Marele, P. De Padova, I. Montero, F. Hennies, A. Pietzsch, M. N. Shariati, J. M. Gómez-Rodríguez and G. LeLay, Nanotechnology, 2012, 23, 385703 CrossRef PubMed.
- T. H. Osborn, A. A. Farajian, O. V. Pupyesheva, R. S. Aga and L. L. Y. Voon, Chem. Phys. Lett., 2011, 511, 101–105 CrossRef CAS.
- S. Zhong, F. Ning, F. Rao, X. Lei, M. Wu and L. Zhou, Int. J. Mod. Phys. B, 2016, 1650176 CrossRef.
- K. K. Paulla and A. A. Farajian, J. Phys. Chem. C, 2013, 117, 12815–12825 CAS.
- M. Y. Han, B. Özyilmaz, Y. B. Zhang and P. Kim, Phys. Rev. Lett., 2007, 98, 206805 CrossRef PubMed.
- Z. Chen, Y.-M. Lin, M. J. Rooks and P. Avouris, Phys. E, 2007, 40, 228–232 CrossRef CAS.
- L. B. Luo, X. B. Yang, F. X. Liang, J. S. Jie, C. Y. Wu, L. Wang, Y. Q. Yu and Z. F. Zhu, J. Phys. Chem. C, 2011, 115, 24293–24299 CAS.
- J. S. Park, B. Ryu, C. Y. Moon and K. J. Chang, Nano Lett., 2010, 10, 116–121 CrossRef CAS PubMed.
- L. B. Luo, X. B. Yang, F. X. Liang, H. Xu, Y. Zhao, X. Xie, W. F. Zhang and S. T. Lee, J. Phys. Chem. C, 2011, 115, 18453–18458 CAS.
- K.-H. Hong, J. Kim, J. H. Lee, J. Shin and U. I. Chung, Nano Lett., 2010, 10, 1671–1676 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21293j |
| This journal is © The Royal Society of Chemistry 2016 |