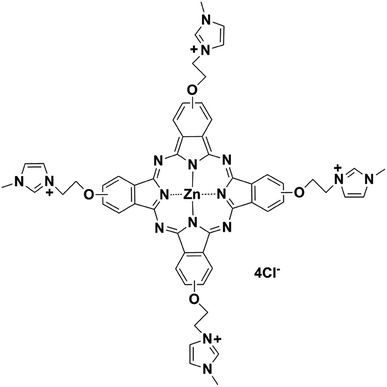
Significant photoelectric conversion properties of multilayer films formed by a cationic zinc phthalocyanine complex and graphene oxide†
Wei Zhang,
Guoqin Li,
Xiaoying Fei,
Yu Zhang,
Jian Tong and
Xi-Ming Song*
Liaoning Key Laboratory for Green Synthesis and Preparative Chemistry of Advanced Materials, College of Chemistry, Liaoning University, Shenyang 110036, China. E-mail: songlab@lnu.edu.cn; Fax: +86-24-62202380; Tel: +86-24-62207792
First published on 7th July 2016
Abstract
A novel kind of multilayer films consisting of graphene oxide and a cationic zinc phthalocyanine complex (ImZnPc) were fabricated through an electrostatic layer-by-layer self-assembly technique and were characterized using UV-vis absorption spectroscopy, X-ray photoelectron spectroscopy and scanning electron microscopy. These multilayer films were not only homogeneously formed, but also the complex ImZnPc was absorbed on the surface of GO as a non-aggregative monomolecular film. Under visible light irradiation, these films exhibited significant cathodic photocurrent generation with good stability and reproducibility. Especially, the four-layer film exhibited a very large cathodic photocurrent density of 5.6 μA cm−2, which makes this novel hybrid thin film a potential material for photoelectric conversion.
1 Introduction
Graphene, a single layer of sp2-bonded carbon atoms, is an attractive material for various applications because of its unique electronic and optical properties.1–4 Graphene oxide (GO) could be regarded as graphene functionalized by carboxylic acid, hydroxyl, and epoxide groups.5–7 The presence of these covalent oxygen functional groups in GO gives rise to the high hydrophilicity and rich chemical reactivity of GO and makes it easy to further be modified by functional molecules, forming different composites and strongly tuning the electrical, optical and optoelectronic properties of GO.8–12 The hydrophilic characteristic of GO make it readily dispersed in a variety of solvents, including water, as individual sheets to form stable colloidal suspensions. These fluid suspensions could be readily used to deposit GO-based thin films that hold promising potential for applications in GO-based thin-film electronics and optoelectronics.13–16Layer-by-layer (LBL) self-assembly technique is based on electrostatic interactions of positively and negatively charged polyelectrolytes, which possesses many advantages such as the experimental simplicity, versatility, low cost, as well as the possibility to control film thickness and architecture at molecular scale.17 In recent years, LBL technique has been applied to fabricate GO-based multilayer films by taking advantage of GO's negatively charged surface in aqueous solution.18–22 The obtained GO-based multilayer films containing photoactive complexes, such as ruthenium(II) complex20,21 and cobalt complex,22 exhibited large photocurrent density and made them have the promising applications as photovoltaic and photocatalytic materials.
Metallo-phthalocyanines (MPcs) have been widely studied due to their unique electronic and optical properties, high thermal and chemical stability and low cost.23–29 Their applications as a photoactive component in organic electronic devices, organic solar cells usually require them to be used as a thin film deposited on a solid substrate, but their strong aggregation behaviors usually decrease their optoelectronic properties in the film and also hinder the fabrication of their homogeneous films. The LBL assembly method has also been utilized to prepare the multilayer films which contained GO and anionic water soluble MPcs such as tetrasulfonated MPcs and their applications as sensors or biosensors have been studied.30–32 But in these films, in order to realize the electrostatic assembly of GO and anionic tetrasulfonated MPcs, cationic polyelectrolytes, such as poly(diallyldimethylammonium chloride) (PDDA) and polycation chloride 3-n-propylpyridinium polymer (SiPy+Cl−) etc., must be used as a stabilizer to modify the surface of GO and make GO positively charged firstly. As we known, no GO/MPcs multilayer film without stabilizers and the photoelectric conversion properties of the GO/MPcs multilayer films have been reported. In this work, we prepared a novel GO/MPcs multilayer film without any extra stabilizer through using cationic zinc(II)-phthalocyanine and also studied its photoelectric conversion property systematically.
2 Experimental section
2.1 Materials and methods
(3-Aminopropyl)trimethoxysilane (99%) was purchased from Acros and used without further purification. Graphene oxide suspension was prepared by sonication of graphite oxide powder produced according to the procedure reported in our previous publication.12 The preparation of water-soluble 2(3),9(10),16(17),23(24)-tetrakis{2-[N′-(N-methyl-imidazole)]ethoxyphthalocyaninato}zinc(II)chloride (ImZnPc) was described in ESI, Scheme S1,† whose molecular structure is shown in Scheme 1. Other materials were commercially available and used without further purification.1H-NMR was recorded on a Varian Mercury 400 MHz spectrometer using TMS as internal reference. Mass spectra were provided by Q-Tof micro (Micromass Int., Monchester, England). XPS data was obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W AlKα radiation. The XPS data were fitted by XPS peak and further plotted by OriginPro 7.0. Electronic spectra in the UV-vis region were recorded with an Agilent L35-900 UV-vis spectrophotometer. X-ray diffraction patterns were recorded by a Bruker (Germany) D8 Advance diffractometer with CuKα radiation (1.54056; 60 kV, 80 mA) in the range of 20–80° (2θ) at a scanning rate of 12° min−1. The morphologies of the samples were observed on a transmission electron microscopic (TEM, HITACHI H-7650 microscope). The surface morphologies of the deposited films were studied using scanning electron microscopy (SEM; Hitachi SU-8010). The photocurrent measurements were recorded by electrochemistry workstation system (CHI660, China) with a Xe lamp (CHFXM500, Beijing Trusttech Co. Ltd) as light source.
2.2 Preparation of [GO/ImZnPc]n multilayer films
The multilayer films were fabricated with a layer-by-layer (LBL) assembly technique using negatively charged GO and positively charged ImZnPc (see Scheme S1 in ESI†). Prior to film preparation, the quartz or ITO substrates were precleaned by sonicating in detergent, acetone and deionized water for 5 min each. Then the quartz slides were immersed in a freshly prepared piranha solution (concentrated H2SO4 (98 wt%)/H2O2 (30 wt%) = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol/vol) for 40 min at 70 °C and the ITO slides were immersed in a solution of a 1
1 vol/vol) for 40 min at 70 °C and the ITO slides were immersed in a solution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v/v mixture of NH3·H2O (25%), H2O2 (30%) and deionized water for 40 min at 70 °C. After being rinsed several times with high-purity water and ethanol and dried by nitrogen gas, the quartz or ITO substrates were cured in a drying oven at 100 °C for 30 min. The silanization was carried out by inserting glass slides in a 3-aminopropyltriethoxysilane/deionized water/ethanol (2
5 v/v/v mixture of NH3·H2O (25%), H2O2 (30%) and deionized water for 40 min at 70 °C. After being rinsed several times with high-purity water and ethanol and dried by nitrogen gas, the quartz or ITO substrates were cured in a drying oven at 100 °C for 30 min. The silanization was carried out by inserting glass slides in a 3-aminopropyltriethoxysilane/deionized water/ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) solution for 12 h at 35 °C to produce amino group-covered surfaces. Then, the glass slides were washed with ethanol, dried by nitrogen gas, and put into a 120 °C oven for 30 min. The amino groups covered on the surface were protonated by immersing the silanized sheets in an aqueous HCl solution (pH ≈ 3) for 10 min, followed by thorough washing with high-purity water.
95, v/v) solution for 12 h at 35 °C to produce amino group-covered surfaces. Then, the glass slides were washed with ethanol, dried by nitrogen gas, and put into a 120 °C oven for 30 min. The amino groups covered on the surface were protonated by immersing the silanized sheets in an aqueous HCl solution (pH ≈ 3) for 10 min, followed by thorough washing with high-purity water.
Subsequently, a piece of clean ITO (or quartz slide) was immersed into a 1 mg mL−1 GO aqueous solution for 10 min, rinsed with high-purity water, dried with N2, and then immersed in a 0.5 mM ImZnPc aqueous solution for 30 min. This procedure gave a single deposition cycle and the obtained film was called one-layer film and marked as ITO/(GO/ImZnPc)1. This one-layer film was immersed into a 1 mg mL−1 GO aqueous solution again and rinsed with high-purity water, dried with N2. Now the obtained film was marked as ITO/(GO/ImZnPc)1GO. After it was immersed in a 0.5 mM ImZnPc aqueous solution again, the ITO/(GO/ImZnPc)2 film was obtained. The multilayered films of quartz or ITO/(GO/ImZnPc)n were obtained by repeating the above-mentioned two steps alternatively (n stands for the number of layer on both sides of the substrate).
3 Results and discussion
3.1 Materials characterization
The cationic phthalocyanine ImZnPc with Cl− as its counter ions was prepared according to a modified procedure previously reported for ImZnPc with I− as the counter ions.33 In literature, cationic water soluble Pcs were generally synthesized by two steps: firstly the water insoluble Pcs peripherally containing imidazole, pyridyl or tertiary amino moieties were prepared and then the water soluble Pcs can be obtained by quaternization of the former with excess alkyl iodide as quaternization agent.34,35 While in this study, ImZnPc was directly synthesized through cyclotetramerization reactions using a dicyano compound containing imidazole cation as precursors (Scheme S1†), and their 1H NMR resonance peaks can be unambiguously assigned according to the protons in the structure of ImZnPc shown in Fig. S1.† The TOF mass spectrum of ImZnPc displays [(M − 2Cl)/2]+, [(M − 3Cl)/3]+, [(M − 4Cl)/4]+ peaks at m/z of 573.9, 370.9, 269.5 respectively, which are all consistent with their theoretical values.The obtained water-soluble GO sheets were characterized by X-ray diffraction (XRD), transmission electron microscope (TEM) and zeta potential analysis (see ESI, Fig. S2–S4†). The XRD analysis exhibits that the interlay space of GO (2θ = 10.7°, 0.828 nm) is larger than that of the pure graphite (2θ = 26.6°, 0.335 nm), as a result of the introduction of oxygenated functional groups on carbon sheets, which agrees with the interlayer spacing of the GO sheets reported in the literature.36 Corrugated GO sheets overlapping each other can be readily observed in the TEM (Fig. S3†) because the electronic repulsion between layers makes the soft and flexible sheets form the bumps and wrinkles. In order to determine the type of the charge on the GO surface, the zeta-potential of GO sheets were measured in PBS (pH = 7.0) (see Fig. S4†). The result showed that the surface of GO was negatively charged and its zeta-potential was found at −26.3 mV around. Therefore, in theory, the (GO/ImZnPc)n multilayer films can be fabricated with a LBL assembly technique using negatively charged GO and positively charged ImZnPc.
3.2 Films characterization
The surface morphology of bare ITO, ITO/(GO/ImZnPc)4GO, ITO/(GO/ImZnPc)5 films were investigated using scanning electron microscope (SEM). From Fig. 1a, it can be seen that the bare ITO shows an irregular and rough surface which is composed of many grains with variable shapes and sizes. While in Fig. 1b, fuzzy grains can be found on the surface of ITO/(GO/ImZnPc)4GO films due to the alternate adsorption of GO and ImZnPc on the surface of ITO and the wrinkles of the surface indicate the deposition of GO nanosheets as the outermost layer. By contrast, the surface of the ITO/(GO/ImZnPc)5 film with ImZnPc (Fig. 1c) as the outermost layer exhibits a relatively flat and homogeneous morphology, which can be explained that the wrinkles of GO were covered by the adsorption of ImZnPc molecules.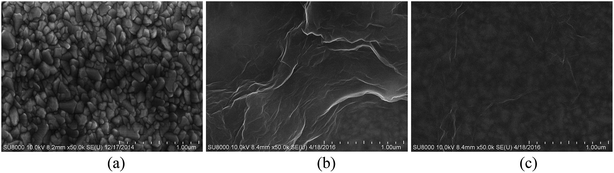 | ||
Fig. 1 Scanning electron micrographs of (a) bare ITO, (b) ITO/(GO/ImZnPc)4GO film and (c) ITO/(GO/ImZnPc)5 film at top view under 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification. 000 magnification. | ||
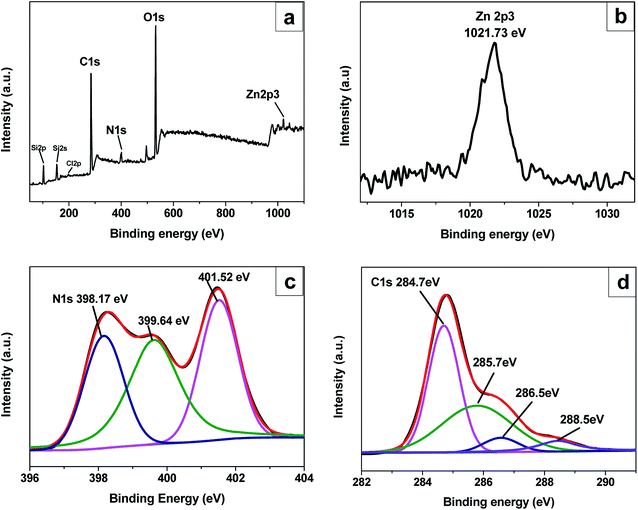
The elemental compositions in the self-assembled films were evaluated by X-ray photoelectron spectroscopy, and the full-range X-ray photoelectron spectrum for ITO/(GO/ImZnPc)5 film is shown in Fig. 2a. As shown in Fig. 2a, the characteristic photoelectron peaks for Zn 2p and N 1s originated from ImZnPc and that for C 1s from GO indicate that ImZnPc and GO were transferred into the film. The presence of Cl 2p weak signal at 197.54 eV from the counter ion of the ImZnPc indicates that the π–π interaction between GO and ImZnPc is another driving force for film formation besides the electrostatic attraction between ImZnPc cation and GO anion.37 By close inspection of Zn 2p, N 1s and C 1s in Fig. 2b–d, the Zn 2p3/2 characteristic photoelectron peak was found to appear at 1021.73 eV, which is the same as the Zn 2p3/2 photoelectron peak at 1021.7 eV previously reported for a protoporphyrin IX zinc (ZnPP) covalently appended monolayer film on a glass substrate;38 the N 1s XPS spectrum clearly indicates that the peaks of nitrogen functionalities appeared at 398.17 eV (the N in C–N bonds) and 401.52 eV (the N in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds) of ImZnPc, in addition at 399.64 eV attributed to the amino group on the ITO surface;39 and the C 1s band can be deconvoluted into the four components centered at 284.7 eV, 286.5 eV, 288.5 eV, and 285.7 eV, where the former three peaks can be assigned to C–C, C–O and C
N bonds) of ImZnPc, in addition at 399.64 eV attributed to the amino group on the ITO surface;39 and the C 1s band can be deconvoluted into the four components centered at 284.7 eV, 286.5 eV, 288.5 eV, and 285.7 eV, where the former three peaks can be assigned to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O respectively,40 while the latter component of C–N, which the pure GO did not contain, is attributed to the macrocycles of ImZnPc.
O respectively,40 while the latter component of C–N, which the pure GO did not contain, is attributed to the macrocycles of ImZnPc.
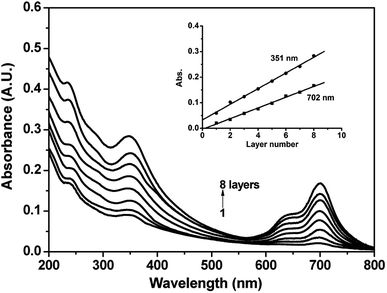
Fig. 3 shows the UV-vis absorption spectra of the quartz/(GO/ImZnPc)8 film, the GO aqueous solution, ImZnPc in methanol and in water respectively. The GO aqueous solution exhibits a band at 239 nm that is ascribed to the π–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in GO.36 ImZnPc in methanol exhibits its characteristic absorptions in the Q band region at 674 nm and B band absorptions at 348 nm. Aggregation (especially in aqueous media) is a very common phenomenon for the family of Pcs, which is usually depicted as a co-planar association of rings progressing from the monomer to dimer and higher order complexes and affects the shape of the Q band. ImZnPc in water shows a typical appearance of a new band around 636 nm at the blue side of the main Q band at 674 nm, which suggests clearly the presence of aggregated species.41 In the case of the quartz/(GO/ImZnPc)8 film, two characteristic absorption peaks at 351 nm and 702 nm should be assigned to the B band and Q band absorption of ImZnPc in the film respectively, and the appearance of the absorption band from 550 to 750 nm illustrates that the aggregation of the ImZnPc molecules in water have been effectively relieved after being absorbed onto the surface of GO and the ImZnPc molecules stayed mainly as monomer in the film;42–44 the absorption at 239 nm in the film could be easily recognized as the contribution from the absorption of GO, which further confirms that both the GO and ImZnPc were deposited into the films. Moreover, the Q band absorption peak of ImZnPc in the multilayer film is found to be bathochromically shifted by 28 nm compared to that of monomer ImZnPc in methanol, indicating that not only electrostatic attraction but also strong π–π interaction existed between GO and ImZnPc. These results are coincided with the analysis of the XPS spectra.
C bonds in GO.36 ImZnPc in methanol exhibits its characteristic absorptions in the Q band region at 674 nm and B band absorptions at 348 nm. Aggregation (especially in aqueous media) is a very common phenomenon for the family of Pcs, which is usually depicted as a co-planar association of rings progressing from the monomer to dimer and higher order complexes and affects the shape of the Q band. ImZnPc in water shows a typical appearance of a new band around 636 nm at the blue side of the main Q band at 674 nm, which suggests clearly the presence of aggregated species.41 In the case of the quartz/(GO/ImZnPc)8 film, two characteristic absorption peaks at 351 nm and 702 nm should be assigned to the B band and Q band absorption of ImZnPc in the film respectively, and the appearance of the absorption band from 550 to 750 nm illustrates that the aggregation of the ImZnPc molecules in water have been effectively relieved after being absorbed onto the surface of GO and the ImZnPc molecules stayed mainly as monomer in the film;42–44 the absorption at 239 nm in the film could be easily recognized as the contribution from the absorption of GO, which further confirms that both the GO and ImZnPc were deposited into the films. Moreover, the Q band absorption peak of ImZnPc in the multilayer film is found to be bathochromically shifted by 28 nm compared to that of monomer ImZnPc in methanol, indicating that not only electrostatic attraction but also strong π–π interaction existed between GO and ImZnPc. These results are coincided with the analysis of the XPS spectra.
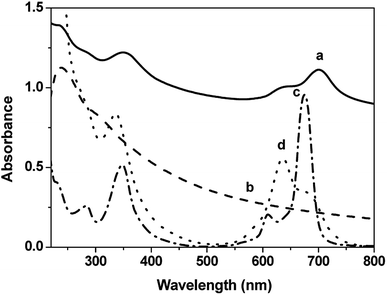 | ||
| Fig. 3 UV-vis absorption spectra of the quartz/(GO/ImZnPc)8 film (a), the GO aqueous solution (b), ImZnPc (6 × 10−6 mol L−1) in methanol (c) and ImZnPc (6 × 10−6 mol L−1) in water (d). | ||
Film growth was also monitored by UV-vis spectroscopy, as shown in Fig. 4. As illustrated in the figure, the film absorption increases with increasing deposition cycles from n = 1 to 8. In particular, both the absorbances of the films located at 351 and 702 nm are linearly proportional to the number of bilayers, which indicates that an equal amount of ImZnPc and GO were transferred into the films during each deposition cycle.
The surface density (mol cm−2) of the complex in the film was calculated by using eqn (1) which is derived from the Beer–Lambert law and modified for two-dimensional concentration.45,46
| Γ = 10−3D/ε | (1) |
In this equation, Γ is the two-dimensional concentration of the ImZnPc monomer in one-layer GO/ImZnPc film; ε is the molar absorption coefficient (L mol−1 cm−1) of the ImZnPc monomer in the film, which is approximately taken to be the ε value in methanol, 155844 L mol−1 cm−1; D is the absorbance of the ImZnPc monomer in one-layer GO/ImZnPc film. By using the slope value 0.02045 of the straight line (702 nm) as the absorbance value at 702 nm for one-layer GO/ImZnPc film on the both sides of quartz and ignoring the absorbance of the GO in one-layer GO/ImZnPc film, the D value is validly derived to be 0.01023. A mean Γ value of ImZnPc in the multilayers was calculated to be 6.56 × 10−11 mol cm−2 and an apparent molecular area for per ImZnPc molecule is derived to be 2.45 nm2, which is larger than the cross-sectional areas of the phthalocyanine ring (ca. 2 nm2). These data indicate that a non-aggregative monomolecular ImZnPc film was formed on the GO surface, which is coincided with the analysis of the UV-vis spectra. The formation of the phthalocyanine monomolecular film can prevent the intermolecular self-quenching efficiently and improve the photoelectrochemical properties of the (GO/ImZnPc)n films.
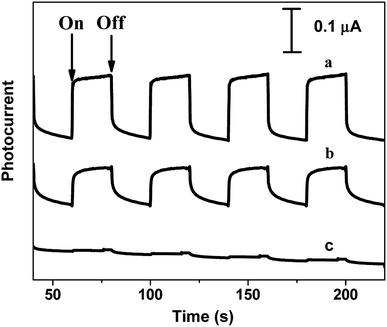
The photocurrent generated from bare ITO, ImZnPc-free monolayer GO and monolayer (GO/ImZnPc)1 film-covered ITO electrodes were studied under 100 mW cm−2 simulated sunlight irradiation at an applied potential of −0.2 V versus SCE in 0.1 M Na2SO4 aqueous solution firstly. As shown in Fig. 5, the bare ITO exhibited negligible photocurrent signals. In contrast, monolayer GO-covered ITO and monolayer ITO/(GO/ImZnPc)1 film exhibited stable and prompt cathodic photocurrents, which could be generated reversibly and repeatedly under several on/off cycles of irradiation. The photocurrent of the ITO/(GO/ImZnPc)1 film was enhanced by ∼100% with respect to the GO monolayer film, indicating that the introduction of the ImZnPc layer significantly enhanced the photocurrent density of the GO layer.
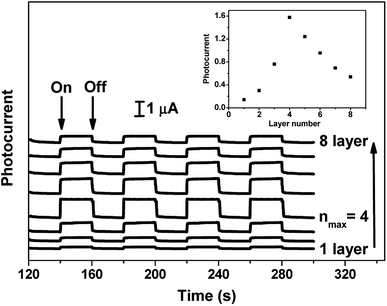
In order to determine the optimal layer number of the film for photoelectric conversion, the relationship between photocurrent values and the numbers of layers was studied. As illustrated in Fig. 6, the cathodic photocurrents for ITO/(GO/ImZnPc)n (n = 1–8) films increased with increasing the number of the layers deposited up to 4 layers and the maximum photocurrent density for (GO/ImZnPc)4 film was found to be 5.6 μA cm−2, which suggests that the electron transport in this film is very efficient. However, when the number of layers was 5 or more, the photocurrent showed a decreasing trend, which is because the increase in the light-harvesting capacity of the film was offsetted by the resistance increase in the electron transport across the layers after the film became relatively thicker.47,48 Additionally, it is noteworthy that the magnitude of the photocurrent generated by the (GO/ImZnPc)4 film without noble metals compares favourably with those previously observed for the polypyridine ruthenium complex-related LBL thin film materials {e.g., 4.1 μA cm−2 for (GO/[Ru(bpy)2L]2+)4 (L = 2-(2,6-di(pyridine-2-yl)pyridine-4-yl)-1H-imidazo[4,5-f]-1,10-phenanthroline)21 and 4.09 μA cm−2 for (GO/RuTTP)2 (TTP = 4′-(thiophen-2-yl)-2,2′:6′,2′′-terpyridine)20}. This result suggests that this film can act as light-harvesting material involved in the photoelectric conversion process.
The photocurrent polarity was further determined through studying the effects of bias voltages and the presence of electron donors or acceptors in the electrolyte solution on the photocurrents. As shown in Fig. 7, when the bias voltages became more negative, the photocurrents were more enhanced. While positive bias voltages were applied, the voltages had little effect on the photocurrents. This behaviors are similar to the previously reported thin films which exhibited cathodic photocurrent generation49 and also indicate that the electrons flow from the ITO electrode through the (GO/ImZnPc)n film to the electrolyte solution. The cathodic photocurrent was also confirmed by the significant decrease of the photocurrent after the addition of an electron donor of hydroquinone (HQ) in the electrolyte solution (see Fig. S5†) or after the partial removal of the electron acceptor oxygen by deoxygenation of the electrolyte solution with nitrogen (see Fig. S6†), because O2 as an electron acceptor in solution could accept an electron to form a superoxide anion and thus enhanced the cathodic photocurrents in similar photoelectrochemical cell.50–53
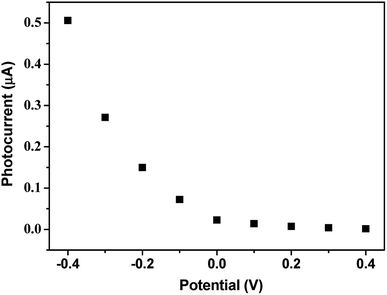 | ||
| Fig. 7 Dependence of the photocurrents of (GO/ImZnPc)1 film on the applied potentials. Conditions: 100 mW cm−2 simulated sunlight; electrode area = 0.25 cm2; supporting electrolyte: 0.1 M Na2SO4. | ||
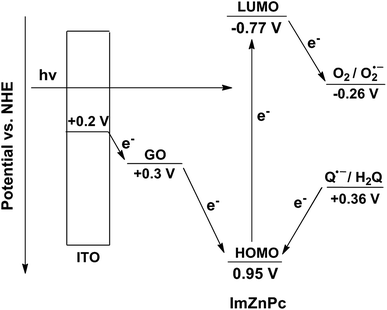
The possible mechanism of cathodic photocurrent generation is proposed in Scheme 2, which involves the energy level arrangements for the species related to the photocurrent generation. The energy of ITO electrode's conduction band, the working function value of GO, the reduction potential of O2 and the oxidation potential of H2Q are taken to be +0.2,54 +0.3,54 −0.26,55 and +0.36 V (ref. 56) vs. NHE respectively according to literature. The energy values of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of ImZnPc were calculated using UV-vis absorption spectral and cyclic voltammetry data. From an onset oxidation potential Eonset(ox) at 0.71 V vs. SCE (0.95 V vs. normal hydrogen electrode, NHE) of ImZnPc in cyclic voltammetry curve (Fig. S7†), the energy of HOMO for ImZnPc was derived to be −5.45 eV vs. vacuum (0.95 V vs. NHE). The excitation transition energy E0–0 (E0–0 = 1240/λonset) value was roughly estimated to be 1.72 eV (the onset absorption value is 720 nm for the complex in (GO/ImZnPc)1 film in UV-vis absorption spectrum). Therefore, ELUMO was calculated to be −3.73 eV vs. vacuum (−0.77 V vs. NHE) according to eqn (2) and (3) by using an energy of −4.50 eV for NHE with respect to the zero vacuum level.21,57
| EHOMO (eV) = −4.50 − Eonset(ox) | (2) |
| ΔE0–0 = ELUMO − EHOMO = 1240/λonset | (3) |
Upon illumination, the electrons of ImPcZn molecules were excited from the ground state (HOMO) to the excited state (LUMO), and the excited state ImPcZn* molecules simultaneously gave the electrons to electron acceptor O2 in the electrolyte solution. To complete the circuit, the excited state ImPcZn* must be regenerated by the electron transfer from GO to the hole residing in the ground state of the ImPcZn and then GO which acted as an electron shuttle accepted the electrons from the conductive band of the ITO electrode. Consequently, the cathodic photocurrent was produced. However, when hydroquinone molecules dissolved in the electrolyte solution, they would compete with the intermolecular electron transfer from GO to ImPcZn and also give electrons to ImPcZn molecules, therefore leading to the decrease of the cathodic photocurrents.
4 Conclusions
(GO/phthalocyanine)n multilayer LBL films were prepared successfully through using a cationic water soluble phthalocyanine complex ImPcZn without adding any extra stabilizer by the electrostatic self-assembly technique. X-ray photoelectron spectroscopy, UV/vis absorption spectroscopy and scanning electron microscopy showed that the self-assembled films were uniformly formed by the alternate deposition and especially the phthalocyanine molecules were deposited as a monomolecular film on the GO surface. This multilayer films showed excellent photoelectric conversion properties with a very large cathodic photocurrent density of 5.6 μA cm−2 for a four-layer film attributing to the light-harvesting role of ImPcZn and GO as well as the electron shuttle role of GO in the film. The novel kind of films has potential applications in developing photovoltaic conversion devices as well as photocatalytic molecular devices.Acknowledgements
The authors are grateful to the Natural Science Foundation of China (No. 21203082, 51273087), Natural Science Foundation of Liaoning Province (Science and Technology Department) (No. 201102084) for financial support of this work.Notes and references
- H. B. Heersche, P. Jarillo-Herrero, J. B. Oostinga, L. M. K. Vandersypen and A. F. Morpurgo, Nature, 2007, 446, 56–59 CrossRef CAS PubMed.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- D. Li and R. B. Kaner, Science, 2008, 320, 1170–1171 CrossRef CAS PubMed.
- X. Zhou, X. Huang, X. Qi, S. Wu, C. Xue, F. Y. C. Boey, Q. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CAS.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed.
- D. W. Boukhvalov and M. I. Katsnelson, J. Am. Chem. Soc., 2008, 130, 10697–10701 CrossRef CAS PubMed.
- L. Liu, S. Ryu, M. R. Tomasik, E. Stolyarova, N. Jung, M. S. Hybertsen, M. L. Steigerwald, L. E. Brus and G. W. Flynn, Nano Lett., 2008, 8, 1965–1970 CrossRef CAS PubMed.
- C. Chen, W. Cai, M. Long, B. Zhou, Y. Wu, D. Wu and Y. Feng, ACS Nano, 2010, 4, 6425–6432 CrossRef CAS PubMed.
- T. F. Yeh, F. F. Chan, C. T. Hsieh and H. Teng, J. Phys. Chem. C, 2011, 115, 22587–22597 CAS.
- Y. Xu, Z. Liu, X. Zhang, Y. Wang, J. Tian, Y. Huang, Y. Ma, X. Zhang and Y. Chen, Adv. Mater., 2009, 21, 1275–1279 CrossRef CAS.
- W. Zhang, H. Bai, Y. Zhang, Y. Sun, S. Lin, J. Liu, Q. Yang and X.-M. Song, Mater. Chem. Phys., 2014, 147, 1140–1145 CrossRef CAS.
- Y. Liu, D. Yu, C. Zeng, Z. Miao and L. Dai, Langmuir, 2010, 26, 6158–6160 CrossRef CAS PubMed.
- H. Li, J. Chen, S. Han, W. Niu, X. Liu and G. Xu, Talanta, 2009, 79, 165–170 CrossRef CAS PubMed.
- K. K. Manga, Y. Zhou, Y. Yan and K. P. Loh, Adv. Funct. Mater., 2009, 19, 3638–3643 CrossRef CAS.
- X. Wan, Y. Huang and Y. Chen, Acc. Chem. Res., 2012, 45, 598–607 CrossRef CAS PubMed.
- C. Santos, R. Ferreira, C. Calixto, J. Rufino, J. Garcia, S. Fujiwara, K. Wohnrath and C. Pessoa, J. Appl. Electrochem., 2014, 44, 1047–1058 CrossRef CAS.
- H. Li, S. Pang, S. Wu, X. Feng, K. Müllen and C. Bubeck, J. Am. Chem. Soc., 2011, 133, 9423–9429 CrossRef CAS PubMed.
- D. Huang, J. Lu, S. Li, Y. Luo, C. Zhao, B. Hu, M. Wang and Y. Shen, Langmuir, 2014, 30, 6990–6998 CrossRef CAS PubMed.
- Y. C. Dai, W. Yang, X. Chen, L. H. Gao and K. Z. Wang, Electrochim. Acta, 2014, 134, 319–326 CrossRef CAS.
- T. T. Meng, Z. B. Zheng and K. Z. Wang, Langmuir, 2013, 29, 14314–14320 CrossRef CAS PubMed.
- X. Chen, Y. C. Dai, Z. B. Zheng and K. Z. Wang, J. Colloid Interface Sci., 2013, 402, 107–113 CrossRef CAS PubMed.
- C. C. Lenznoff and A. B. Lever, Properties and Applications, 1989/1993/1996, vol. 1–4 Search PubMed.
- G. de la Torre, C. G. Claessens and T. Torres, Chem. Commun., 2007, 2000–2015 RSC.
- V. Gadenne, M. Bayo-Bangoura, L. Porte and L. Patrone, J. Colloid Interface Sci., 2011, 359, 47–55 CrossRef CAS PubMed.
- S. M. O'Flaherty, L. Wiegart, O. Konovalov and B. Struth, Langmuir, 2005, 21, 11161–11166 CrossRef PubMed.
- C. Schünemann, C. Elschner, A. A. Levin, M. Levichkova, K. Leo and M. Riede, Thin Solid Films, 2011, 519, 3939–3945 CrossRef.
- Q. Wang, W. M. Campbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397–15409 CrossRef CAS PubMed.
- J. Aimi, M. Komura, T. Iyoda, A. Saeki, S. Seki, M. Takeuchi and T. Nakanishi, J. Mater. Chem. C, 2015, 3, 2484–2490 RSC.
- L. Cui, T. Pu, Y. Liu and X. He, Electrochim. Acta, 2013, 88, 559–564 CrossRef CAS.
- Y. Q. Zhang, Y. J. Fan, L. Cheng, L. L. Fan, Z. Y. Wang, J. P. Zhong, L. N. Wu, X. C. Shen and Z. J. Shi, Electrochim. Acta, 2013, 104, 178–184 CrossRef CAS.
- R. B. Estevam, R. T. Ferreira, A. B. H. Bischof, F. S. dos Santos, C. S. Santos, S. T. Fujiwara, K. Wohnrath, S. R. Lazaro, J. R. Garcia and C. A. Pessoa, Surf. Coat. Technol., 2015, 275, 2–8 CrossRef CAS.
- Y. Ge, X. Weng, T. Tian, F. Ding, R. Huang, L. Yuan, J. Wu, T. Wang, P. Guo and X. Zhou, RSC Adv., 2013, 3, 12839–12846 RSC.
- H. Li, T. J. Jensen, F. R. Fronczek and M. G. H. Vicente, J. Med. Chem., 2008, 51, 502–511 CrossRef CAS PubMed.
- H. R. P. Karaoğlu, A. Koca and M. B. Koçak, Dyes Pigm., 2012, 92, 1005–1017 CrossRef.
- S. Min and G. Lu, J. Phys. Chem. C, 2011, 115, 13938–13945 CAS.
- X. Zhang, Y. Feng, S. Tang and W. Feng, Carbon, 2010, 48, 211–216 CrossRef CAS.
- Z. Zhang, R. Hu and Z. Liu, Langmuir, 1999, 16, 1158–1162 CrossRef.
- J. Zhu, Y. Li, Y. Chen, J. Wang, B. Zhang, J. Zhang and W. J. Blau, Carbon, 2011, 49, 1900–1905 CrossRef CAS.
- Y. Yu, M. Zhou, W. Shen, H. Zhang, Q. Cao and H. Cui, Carbon, 2012, 50, 2539–2545 CrossRef CAS.
- C. C. Leznoff and A. B. P. Lever, Phthalocyanines : properties and applications, VCH, New York, 1989 Search PubMed.
- Z. Bıyıklıoğlu, Synth. Met., 2012, 162, 26–34 CrossRef.
- D. Kessel, J. Porphyrins Phthalocyanines, 2004, 08, 1009–1014 CrossRef CAS.
- Y. Zhang, X. Chen, J. Lan, J. You and L. Chen, Chem. Biol. Drug Des., 2009, 74, 282–288 CAS.
- R. Marczak, V. Sgobba, W. Kutner, S. Gadde, F. D'Souza and D. M. Guldi, Langmuir, 2007, 23, 1917–1923 CrossRef CAS PubMed.
- L. H. Gao, K. Z. Wang, L. Cai, H. X. Zhang, L. P. Jin, C. H. Huang and H. J. Gao, J. Phys. Chem. B, 2006, 110, 7402–7408 CrossRef CAS PubMed.
- K. Jiang, H. Xie and W. Zhan, Langmuir, 2009, 25, 11129–11136 CrossRef CAS PubMed.
- K. Akatsuka, Y. Ebina, M. Muramatsu, T. Sato, H. Hester, D. Kumaresan, R. H. Schmehl, T. Sasaki and M.-a. Haga, Langmuir, 2007, 23, 6730–6736 CrossRef CAS PubMed.
- L. H. Gao, Q. L. Sun, J. M. Qi, X. Y. Lin and K. Z. Wang, Electrochim. Acta, 2013, 92, 236–242 CrossRef CAS.
- A. D. Lang, J. Zhai, C. H. Huang, L. B. Gan, Y. L. Zhao, D. J. Zhou and Z. D. Chen, J. Phys. Chem. B, 1998, 102, 1424–1429 CrossRef CAS.
- C. C. Ju, H. Luo and K. Z. Wang, J. Nanosci. Nanotechnol., 2010, 10, 2053–2059 CrossRef CAS PubMed.
- X. Zou, Y. Fan, M. Y. Zhuang, J. Peng and K. Z. Wang, J. Nanosci. Nanotechnol., 2010, 10, 2203–2207 CrossRef CAS PubMed.
- L. Y. Li, H. Y. Yu, N. Lin, X. Chen, R. Wei and K. Z. Wang, J. Nanosci. Nanotechnol., 2011, 11, 4089–4096 CrossRef CAS PubMed.
- T. Cassagneau, J. H. Fendler, S. A. Johnson and T. E. Mallouk, Adv. Mater., 2000, 12, 1363–1366 CrossRef CAS.
- H. Imahori, H. Norieda, Y. Nishimura, I. Yamazaki, K. Higuchi, N. Kato, T. Motohiro, H. Yamada, K. Tamaki, M. Arimura and Y. Sakata, J. Phys. Chem. B, 2000, 104, 1253–1260 CrossRef CAS.
- L. Sereno, J. J. Silber, L. Otero, M. del Valle Bohorquez, A. L. Moore, T. A. Moore and D. Gust, J. Phys. Chem., 1996, 100, 814–821 CrossRef CAS.
- T. Suresh, G. Rajkumar, S. P. Singh, P. Y. Reddy, A. Islam, L. Han and M. Chandrasekharam, Org. Electron., 2013, 14, 2243–2248 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The preparation of complex ImPcZn and GO. 1H NMR and cyclic voltammograms of compound ImPcZn. XRD, TEM, zeta-potential analysis of GO. Photocurrent responses for ITO/(GO/ImZnPc)1 film electrodes in the presence of the electron donor or the electron acceptor. See DOI: 10.1039/c6ra15856k |
| This journal is © The Royal Society of Chemistry 2016 |