One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli†
Soumen Chandra,
Angshuman Ray Chowdhuri,
Triveni Kumar Mahto,
Arpita Samui and
Sumanta kumar Sahu*
Department of Applied Chemistry, Indian School of Mines, Dhanbad 826004, Jharkhand, India. E-mail: sahu.s.ac@ismdhanbad.ac.in; sumantchem@gmail.com; Fax: +91 326 2307772; Tel: +91 3262235936 Tel: +91 7631042241
First published on 25th July 2016
Abstract
In this paper, we report a one-step strategy to synthesize amikacin modified fluorescent carbon dots (CDs@amikacin) for assaying pathogenic bacteria, Escherichia coli. Amikacin is a well-known aminoglycoside antibiotic but here we used it as a binding ligand towards E. coli. Here the synthesized carbon dots are used to detect E. coli accompanied with a linear range of 3.904 × 105 to 7.625 × 102 cfu per mL as well as a detection limit of 552 cfu per mL. CDs@amikacin were well dispersed in water with an average particle diameter of ∼2.5 nm and exhibited a quantum yield of 12.35% at a excitation wavelength of 340 nm. The synthesis of CDs@amikacin and their use in the detection of E. coli are simple, cheap and effective process. This study is also successfully applied to the sensing of E. coli in different fruit juice samples like apple, pineapple and orange. We believe that this analytical method can be used in the field of public health as well as food safety.
1. Introduction
Escherichia coli (E. coli) is the most common intestinal microorganism of humans and other warm-blooded animals. E. coli may cause intestinal infection including diarrhea, fatigue, abdominal pain and fever. More severe cases can lead to hemolytic uremic syndrome, septicemia, or even kidney failure. Therefore, many researchers have been dedicated to improving the detection of E. coli by simple, sensitive, rapid and economical methods. In the past few decades, a variety of methods including enzyme-linked immunosorbent assays, electrochemical, polymerase chain reaction and fluorescent labeling etc have been developed for the detection of E. coli.1–4 Among them, fluorescent labeling is a very effective method for the detection of E. coli. Recently, Chen et al. developed dye-doped fluorescent silica nanoparticles for the rapid detection of pathogenic E. coli O157:H7 using fluorescence microscopy and flow cytometry.5 Si et al. reported pH-sensitive fluorescent nanoparticles that can be used for the rapid and accurate real time detection of E. coli growth.6 However, synthesis of such fluorescent nanoparticles is a multistep synthetic route and hence not convenient to be used in routine practical application. Thus, a simple, selective and sensitive analytical method for detection of trace E. coli is still required. In this context we have developed surface modified carbon dots based fluorescent nanoparticles for E. coli detection.Now a days, carbon dots (CDs) have been broadly studied in a wide range of applications including bio-imaging,7 drug delivery,8 photodynamic therapy,9 catalysis10 and sensing.11 These CDs exhibit many fascinating optical properties, such as tunable photoluminescence properties,12 low cytotoxicity,13 eco-friendliness,14 and excellent bio-compatibility.15 Apart from the use of CDs for biological labeling and imaging, photocatalysis, and optoelectronic devices, CDs have been employed as fluorescent probe for biological detection owing to their distinct optical features and surface modification.16 In order to expand the areas of application, investigations on CDs surface modification with a variety of surface groups have been carried out by many researchers.17,18 Most of the research papers have focused on bio imaging using CDs but few researchers focused selective detection of bacteria. For example, Weng and co-workers synthesized mannose modified fluorescent carbon quantum dots from ammonium citrate and mannose for labeling E. coli.19 Zhong et al. used CDs modified with vancomycin for assaying Gram-positive bacteria like Staphylococcus aureus.20 Nandi et al. synthesised amphiphilic carbon dots for bacterial detection.21 Bhushan et al. synthesised multifunctional carbon dots for selective labeling of E. coli.22 Here we have tried to diversify the surface group of CDs to enlarge their application in pathogen detection. However, the development of surface modified CDs with high quantum yield is still an interesting challenge.
Amikacin is a synthetic amino glycoside antibiotic. Amino glycosides represent a class of antibiotics with a strong activity against most Gram-negative bacteria and they are widely used in antimicrobial therapy.23 Amikacin treats infections caused by Gram-negative bacteria such as E. coli, Providencia species, indole-positive and indole-negative Proteus species.24,25 Herein, we have employed for the first time, amikacin a biological active molecule conjugated with fluorescent CDs for selective E. coli detection. The detection principle can be explained by the fact that the amikacin modified fluorescence CDs is easy to bind the components of bacteria that produce important bacterial proteins.
In the present study, we have developed a facile and economic synthetic approach for the preparation of CDs via hydrothermal carbonization of amikacin and di ammonium hydrogen citrate for the first time. The formation of CDs and the surface modification by amikacin were accomplished simultaneously in a one pot. The spectral properties and size of the CDs were studied in detail. The fluorescence properties of the as-prepared CDs are examined for the fluorescent detection of E. coli and S. aurous as model Gram-negative and Gram-positive bacteria.
2. Materials and methods
2.1. Materials
Analytical grade reagents without further purification were used throughout the experiments. Di ammonium hydrogen citrate (98%) and di sodium hydrogen phosphate (98.1%) were purchased from Loba Chemie (India). Amikacin hydrate was acquired from Sigma Aldrich. Sodium chloride (≥99%), potassium chloride (99.5%) potassium di hydrogen phosphate (≥98%) and ethyl alcohol were procured from Merck India. Nutrient broth and Luria broth were purchased from Himedia. Millipore water was used for all the experiment. Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria were obtained from Microbiology laboratory of Midnapore Medical College and Hospital (West Bengal). The bacterial cultures were carried out at 37 °C throughout the experiment.2.2. Synthesis of CDs
The CDs were synthesised by the hydrothermal method. In brief, 30 mg of di ammonium hydrogen citrate was dissolved in 10 mL Millipore water to form transparent clear solution. The clear solution of di ammonium hydrogen citrate was transferred to a dried Teflon linked autoclave and kept at 180 °C. After 4 h reaction, the temperature of the autoclave was cooled to room temperature. The brown coloured solution was collected and dialysed with 3500 Da dialysis bag in Millipore water for overnight.2.3. Synthesis of CDs@amikacin
The amikacin conjugated CDs were synthesised by same method by varying the amikacin precursor and these were labeled as CDs@amikacin-1 and CDs@amikacin-2. In CDs@amikacin-1 case 15 mg and CDs@amikacin-2 case 5 mg amikacin were used with 30 mg of di ammonium hydrogen citrate. After synthesis, these amikacin functionalized CDs were treated for bacterial detection.2.4. Bacterial growth
The experiment for selective detection of the bacteria was carried out with Gram-positive (S. aureus) and Gram-negative (E. coli) live bacteria cell. The Gram-positive and Gram-negative bacteria cell were cultured in sterile clean room. All apparatus for bacteria cell culture were sterilized in an autoclave at high temperature and high pressure. The culture of E. coli and S. aureus bacteria were performed separately in sterile Luria–Bertani (LB) media [containing bacto-tryptone (2.0 g), bacto-yeast extract (1.0 g) and NaCl (1.0 g) in deionized water (200 mL). A portion of each colony of E. coli and S. aureus bacteria was lifted from LB agar plates and incubated in 5 mL LB media. The cultures were allowed to grow in an incubator-shaker at 37 °C. After overnight shaking, 1 mL from each cultured media was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the supernatant and washed 4 times with PBS (13.6 mM NaCl, 2.7 mM KCl, 1.003 mM Na2HPO4, 0.176 mM KH2PO4, in 100 mL deionised water; adjusted to pH 7.4 using 0.1 M HCl) and suspended in 1 mL of PBS.
000 rpm for 10 min to remove the supernatant and washed 4 times with PBS (13.6 mM NaCl, 2.7 mM KCl, 1.003 mM Na2HPO4, 0.176 mM KH2PO4, in 100 mL deionised water; adjusted to pH 7.4 using 0.1 M HCl) and suspended in 1 mL of PBS.
2.5. Bacterial assays
Each type of suspended bacteria was again diluted with PBS (pH 7.4) to adjust the absorbance 1.0 at 600 nm wavelength (OD600) and optical path length 1.0 cm. Diluted 1 mL of each type of bacteria were taken as 108 colony forming units (cfu).19 For further experimental study E. coli bacterial cells were again diluted to a concentration of 5 × 107 to 7.625 × 102 cfu per mL. Each 1 mL concentrated (5 × 107 to 7.625 × 102 cfu per mL) E. coli cell were incubated with 0.5 mL CDs@amikacin (1 mg mL−1). Then each mixture was vertexing for 5 minutes and further each mixture were taken under gentle shaking with orbital shaker at 150 rpm for 50 minutes at room temperature. The CDs@amikacin attached E. coli cells were separated from suspension by centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minutes and washed with PBS for further fluorescence study. Finally, the CDs@amikacin attached E. coli cells bacteria pellets were resuspended in PBS and fluorescence spectra were recorded using Perkin Elmer LS 55 Fluorescence Spectrometer, with excitation wavelength of 360 nm.
000 rpm for 15 minutes and washed with PBS for further fluorescence study. Finally, the CDs@amikacin attached E. coli cells bacteria pellets were resuspended in PBS and fluorescence spectra were recorded using Perkin Elmer LS 55 Fluorescence Spectrometer, with excitation wavelength of 360 nm.
2.6. Characterization
The presence of all the functional groups of the precursors as well as CDs@amikacin-1 was investigated by FTIR spectroscopy (Thermo Nicolet Nexus FTIR (model 870)). The size and morphology of the CDs@amikacin-1 were observed by high-resolution transmission electron microscopy (HRTEM) (JEOL 3010, Japan). Emission spectra of CDs@amikacin-1 and CDs were recorded at different excitation by Perkin Elmer LS 55 Fluorescence Spectrometer, 120 V; Perkin-Elmer 950 spectrometer was used to perform UV-VIS absorption spectrum. The attachment of the amikacin to the CDs@amikacin-1 surface was confirmed by FT NMR Spectrometer model Avance-II (Bruker) with frequency 400 MHz. The zeta potentials (ζ) of the CDs@amikacin and CDs were assessed using a Zetasizer (Nano ZS, Malvern Instruments, and Worcestershire, UK).2.7. Quantum yield calculation of synthesized CDs@amikacin
Quantum yield of the synthesised CDs@amikacin-1 and CDs were calculated according to previously reported procedure26 by using quinine sulphate (QY = 0.54) as a standard. Five different concentrations of each CDs@amikacin-1 and CDs were used to estimate the quantum yield of the synthesized CDs@amikacin-1 and CDs. UV-absorbance value of the CDs@amikacin-1, CDs was adjusted to 0.01, 0.02, 0.03, 0.04, 0.05 by UV-VIS spectrometer to prepared different concentrations of CDs@amikacin-1 and CDs in water [refractive index (μ) of 1.33]. Similarly five different concentration of reference was prepared by dissolving quinine sulphate to 0.1 M sulphuric acid [refractive index (μ) of 1.33] to keep the UV-VIS absorbance value approximately 0.01 to 0.05. Finally the fluorescence spectra of five different concentrated solutions of the reference and the CDs@amikacin-1 and CDs were recorded with excitation 340 nm. Successively the integrated photoluminescence intensities of samples and reference were calculated and plotted against UV-VIS absorbance as shown in Fig. S2.† The absolute vales of slope of the plotted straight line for the CDs@amikacin-1, CDs and the reference were determined. Then approximate value of quantum yields of the synthesised CDs@amikacin-1 and CDs was calculated by formula given below.| (QY)s = (QY)st(Ks/Kst)(ηs/ηst)2 | (1) |
2.8. Analysis in real samples
In order to investigate the purpose of practical applicability of CDs@amikacin-1 to the real sample, E. coli was cultured in three different fruit juice. In brief, 0.1 mL suspension of E. coli cultured in LB media was incubated separately in 5 mL of transparent apple, pineapple and orange juice respectively. The cultures were grown in an incubator-shaker at 37 °C to reach the growing stationary phase. After overnight shaking, 1 mL from each cultured suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the supernatant and washed 4 times with PBS (pH 7.4), and resuspended in 1 mL of PBS. The suspended each type of bacterial E. coli was again diluted with PBS to adjust the UV-VIS absorbance 1.0 at 600 nm (OD 600) and optical 1.0 cm path length. For further study E. coli bacterial cells were again diluted to a concentration of 5 × 107 to 7.625 × 102 cfu per mL. Each concentrated E. coli cell were incubated with 0.5 mL amikacin attached CDs (1 mg mL−1) and E. coli cells were suspended by vertexing for 5 minute. The suspended E. coli cells were taken under gentle shaking with orbital shaker at 150 rpm for 50 minutes at room temperature. The CDs@amikacin attached E. coli cells were separated from suspension by centrifuge at 10
000 rpm for 10 min to remove the supernatant and washed 4 times with PBS (pH 7.4), and resuspended in 1 mL of PBS. The suspended each type of bacterial E. coli was again diluted with PBS to adjust the UV-VIS absorbance 1.0 at 600 nm (OD 600) and optical 1.0 cm path length. For further study E. coli bacterial cells were again diluted to a concentration of 5 × 107 to 7.625 × 102 cfu per mL. Each concentrated E. coli cell were incubated with 0.5 mL amikacin attached CDs (1 mg mL−1) and E. coli cells were suspended by vertexing for 5 minute. The suspended E. coli cells were taken under gentle shaking with orbital shaker at 150 rpm for 50 minutes at room temperature. The CDs@amikacin attached E. coli cells were separated from suspension by centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minute and washed with PBS for further fluorescence study. Finally, the CDs@amikacin attached E. coli cells bacteria pellets were resuspended in PBS and fluorescence spectra were recorded using Perkin Elmer LS 55 Fluorescence spectrometer with excitation wavelength of 360 nm.
000 rpm for 15 minute and washed with PBS for further fluorescence study. Finally, the CDs@amikacin attached E. coli cells bacteria pellets were resuspended in PBS and fluorescence spectra were recorded using Perkin Elmer LS 55 Fluorescence spectrometer with excitation wavelength of 360 nm.
3. Results and discussions
Amikacin conjugated CDs can be easily achieved by simple hydrothermal treatment with di ammonium hydrogen citrate and amikacin. Generally, the direct conjugation of a small molecules and CDs could not be easily achieved. Here, amikacin is selected as one of the active molecule and conjugated with fluorescent CDs for selective E. coli detection. Detection procedure by amikacin conjugated CDs is easier and short time taking than other previously works.27–29 Among CDs@amikacin-1 and CDs@amikacin-2, CDs@amikacin-1 showed the very good response towards E. coli detection. Therefore, our detailed study is focused on the CDs@amikacin-1 only.3.1. FTIR study
The functionality of the ammonium citrate, amikacin and CDs@amikacin-1 were studied by FTIR spectroscopy, as shown in Fig. 1(a). FTIR spectra of the synthesized CDs@amikacin-1 clearly identify the carboxylic functionality as well as amide bond at the surface of the dots. The broad band at 3418 cm−1 and 3122 cm−1 indicate the O–H stretching vibration of carboxylic acid functional group as well as N–H stretching vibration respectively.30,31 A sharp peak at 1619 cm−1 at the FTIR spectrum of the CDs@amikacin-1 indicates the carbonyl stretching frequency of amide.32 In the FTIR spectrum of ammonium citrate, peak at 1705 cm−1 indicates the carbonyl stretching frequency of carboxylate ions.33 Decrement of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of CDs@amikacin-1 as compared to carbonyl stretching vibration of ammonium citrate attributed to the formation of amide bond on the surface of the CDs@amikacin-1. Simultaneously, peak at 1405 cm−1 and 1098 cm−1 indicate the starching vibration of C–N bond.34 FTIR spectrum of CDs@amikacin-1 does not clearly confirm the attachment of the amikacin towards the CDs. The attachment of the amikacin to the surface of the CDs was further confirmed by 1H NMR spectroscopy of the CDs@amikacin-1 as well as the precursors.
O stretching vibration of CDs@amikacin-1 as compared to carbonyl stretching vibration of ammonium citrate attributed to the formation of amide bond on the surface of the CDs@amikacin-1. Simultaneously, peak at 1405 cm−1 and 1098 cm−1 indicate the starching vibration of C–N bond.34 FTIR spectrum of CDs@amikacin-1 does not clearly confirm the attachment of the amikacin towards the CDs. The attachment of the amikacin to the surface of the CDs was further confirmed by 1H NMR spectroscopy of the CDs@amikacin-1 as well as the precursors.
3.2. 1H NMR analysis
1H NMR spectra of synthesized CDs@amikacin-1, CDs and amikacin are shown in Fig. S1(a–c).† In the 1H NMR spectrum of CDs@amikacin-1 (Fig. S1c†) the regions found are: 1–3 ppm (for sp3 C–H protons), 3–5 ppm (for the protons attached with carbohydrate groups of amikacin).41,42 The small peak in the region between 3 and 4 ppm demonstrate the presence of amikacin to the surface of the CDs.3.3. Size and morphology study
The particle size and morphology of the synthesised CDs@amikacin-1 were investigated by TEM as shown in Fig. 1(b). TEM image exposes that CDs@amikacin-1 are uniform in size with good spherical morphology. Hundred numbers of CDs@amikacin-1 particles were chosen to draw the particle size distribution curve as shown in Fig. 1(d). From the particle size distribution curve of the CDs@amikacin-1, it was observed that the particles are uniformly dispersed with average particle size ranging from 1.5 nm to 4 nm and maximum CDs@amikacin-1 are 2.5 nm in size. SAED pattern of CDs@amikacin-1 was also shown in Fig. 1(c). There is no diffraction phase is observed in SAED pattern of CDs@amikacin-1, which conformed that the CDs@amikacin-1 particles are amorphous in nature.35 FESEM images of the as synthesised CDs and CDs@amikacin-1 are shown in Fig. S5.† Images clearly revel that CDs and CDs@amikacin-1 are in spherical morphology. EDX spectra and elemental composition of the synthesised CDs and CDs@amikacin-1 are shown in the Fig. S6.† It was observed that CDs@amikacin-1 contained higher nitrogen in compare to CDs. The higher atomic percentage of nitrogen on the synthesised CDs@amikacin-1 revels that some extra nitrogen has been doped from the amikacin.3.4. UV-VIS and fluorescence study
UV-VIS absorption spectra and fluorescence spectra of synthesized CDs@amikacin-1 and CDs without amikacin are shown in Fig. 2. The UV-VIS absorption spectra of CDs@amikacin-1 and CDs in aqueous medium show broad characteristic absorption band at 346 nm and 334 nm respectively. Both these absorption corresponds to n–π* of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O transition.36 The red shifting of absorption band of CDs@amikacin-1 from UV-VIS absorption band CDs due to attachment of amikacin to the CDs surface. The broad peak at 346 nm in the UV absorption spectra of the CDs@amikacin-1 appeared due to trapping of large extent of excitation energy leading to huge amount of emission at 360 nm excitation.37 To study the optical properties of the CDs@amikacin-1 and CDs, detailed photoluminescence (PL) measurements were performed with different excitation wavelengths. Fig. 2(a and c) shows the PL spectra of CDs@amikacin-1 and CDs at different excitation wavelengths starting from 300 nm to 500 nm in which maximum PL intensity was observed at 340 nm excitation wavelength for CDs and 360 nm excitation wavelength for CDs@amikacin-1. The red shifting of the excitation wavelength of CDs@amikacin-1 is observed in compare to CDs. This indicates that some extra nitrogen is doped upon CDs from amikacin site during hydrothermal synthesis.
O transition.36 The red shifting of absorption band of CDs@amikacin-1 from UV-VIS absorption band CDs due to attachment of amikacin to the CDs surface. The broad peak at 346 nm in the UV absorption spectra of the CDs@amikacin-1 appeared due to trapping of large extent of excitation energy leading to huge amount of emission at 360 nm excitation.37 To study the optical properties of the CDs@amikacin-1 and CDs, detailed photoluminescence (PL) measurements were performed with different excitation wavelengths. Fig. 2(a and c) shows the PL spectra of CDs@amikacin-1 and CDs at different excitation wavelengths starting from 300 nm to 500 nm in which maximum PL intensity was observed at 340 nm excitation wavelength for CDs and 360 nm excitation wavelength for CDs@amikacin-1. The red shifting of the excitation wavelength of CDs@amikacin-1 is observed in compare to CDs. This indicates that some extra nitrogen is doped upon CDs from amikacin site during hydrothermal synthesis.
The emission peak of CDs@amikacin-1 shifts from 438 to 552 nm, while the excitation wavelength changes from 300 nm to 500 nm and maximum emission peak centred at 442 nm. Similarly, the emission peak of CDs shifts from 429 to 565 nm, while the excitation wavelength changes from 300 nm to 500 nm and maximum emission peak centred at 440 nm. The full width at half maximum (FWHM) of CDs@amikacin-1 and CDs calculated at 360 nm and 340 nm are about 71 nm and 85 nm. The less value of FWHM of CDs@amikacin-1 compare to FWHM of CDs attributed to the narrower particle size distribution of CDs@amikacin-1.38 The excitation wavelength dependent emission wavelength and intensity of CDs@amikacin-1 are ascribed to quantum effects and emissive traps present on their surface owing to the presence of functional groups.39 At certain excitation wavelengths, some emissive sites would be excited, giving rise to excitation dependent emission spectra.40 Simultaneously, higher value of quantum yield of CDs@amikacin-1 (QY = 12.35%) compare to quantum yield of CDs (QY = 2.41%) indicates that more nitrogen doping was took place during high temperature hydrothermal synthesis.
3.5. Fluorescence stability of CDs@amikacin-1
The effect of PH on the fluorescence intensity of CDs and CDs@amikacin-1 are compared, as shown in the Fig. S3(a).† Result revels that CDs@amikacin-1 has greater fluorescence stability with PH in contrast to CDs. Relatively low fluorescence intensity of CDs compared to CDs@amikacin-1 at very low PH attributed to the higher negatively charge functional group of CDs. This phenomenon was also confirmed by zeta potential measurement of CDs@amikacin-1 and CDs, as shown in the Fig. S4.† Fig. S3(b)† shows the variation of fluorescence intensity of CDs@amikacin-1 with respect to ionic strength of the medium. The fluorescence intensity of CDs@amikacin-1 is also intact in high ionic strength. The effect of irradiation time on the fluorescence intensity of CDs@amikacin-1 was compared with commercial dye quinine, as shown in the Fig. S3(c).† The change of the negligible amount of fluorescent intensity of CDs@amikacin-1 after 140 minute UV light irradiation indicating that CDs@amikacin-1 has greater fluorescence stability compared to commercial dye, quinine. The effect of temperature on the fluorescence intensity of CDs@amikacin-1 and CDs are compared, as shown in the Fig. S3(d).† The result revels that both CDs@amikacin-1 and CDs have same effect on the variation of the temperature.3.6. Zeta potential measurement of CDs@amikacin-1 and CDs
Zeta potentials of CDs@amikacin-1 and CDs are studied at neutral PH, the result corresponding that both CDs@amikacin-1 and CDs have high negative zeta potential. Zeta potentials of CDs@amikacin-1 and CDs are compared as shown in the Fig. S4.† The result revels that the zeta potential of CDs@amikacin-1 slightly decreased due to the passivation of the amikacin to the surface of the CDs.3.7. Detection of E. coli
We have compared the binding efficiency of the two types of CDs@amikacin with series of different concentration of the bacterial E. coli as shown in the Fig. 3. The CDs@amikacin-1 exhibits the more labeling efficiency compared to CDs@amikacin-2. The greater labeling efficiency of CDs@amikacin-1 compared to CDs@amikacin-2 might be attributed to its high content of amikacin molecules on the surface. Simultaneously, a series of different concentration of the bacterial cell of E. coli labeled with CDs@amikacin-1 shows the greater linear relationship with the fluorescence intensity as well as high fluorescence intensity peak as shown in the Fig. 3.The fluorescence intensity of E. coli labeled with CDs@amikacin-1 linearly increases with the increasing concentration of bacterial cell with a limit of detection (LOD; at an S/N ratio of 3) approximately 552 cfu per mL. The fluorescence intensity for the E. coli labeled with CDs@amikacin-2 linearly increases with the increasing concentration of bacterial cell with a limit of detection (LOD; at an S/N ratio of 3) approximately 1023 cfu per mL. The lower value of the LOD for the detection of E. coli was obtained by labeling with CDs@amikacin-1 in compare to CDs@amikacin-2. The result revels that the low contain of amikacin to the surface of the CDs@amikacin-2. The CDs@amikacin-1 exhibits good linear range of detection (3.904 × 105 to 7.625 × 102 cfu per mL) in compared to CDs@amikacin-2 (5 × 107 to 9.76 × 104 cfu per mL). The result of LOD is compared with some other previously reported based optical bacterial sensors.19,43
3.8. Specificity of CDs@amikacin
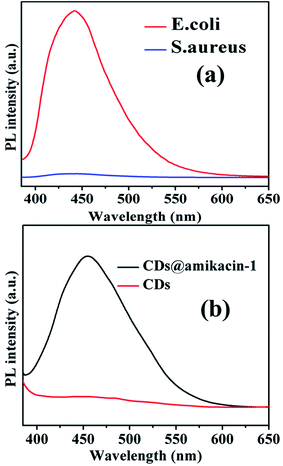
The amikacin unit of the amikacin functionalized CDs binds to the A-site region on the 16S subunit of rRNA.44 To identify the specificity of CDs@amikacin-1 for the detection of E. coli other similar experiment was performed with Gram-positive bacterial cell of S. aureus. The fluorescence intensity of S. aureus bacterial cell was recorded after the treatment with CDs@amikacin-1. Relatively poor fluorescence intensity was observed for S. aureus after the treatment with CDs@amikacin-1. The result revels that the bacterial cell detection by CDs@amikacin-1 was selective only for E. coli.19 Fig. 4(a) represents the fluorescence intensity of E. coli (1 × 108 cfu per mL) and S. aureus (1 × 108 cfu per mL) after the treatment with 0.5 mL CDs@amikacin-1 (1 mg mL−1). Simultaneously, another experiment was conducted with the simple CDs and CDs@amikacin-1 to identify labeling efficiency of E. coli. The fluorescence intensity of E. coli (1 × 108 cfu per mL) bacterial cell was measured after the treatment with CDs. Negligibly small fluorescence intensity peak of E. coli bacterial cell was observed after the treatment with CDs. The result implied that the amikacin conjugated CDs was bind towards E. coli surface. The fluorescence intensities of E. coli (1 × 108 cfu per mL) bacterial cell were recorded separately after treatment with 0.5 mL CDs@amikacin-1 (1 mg mL−1) and 0.5 mL CDs (1 mg mL−1) as shown in the Fig. 4(b).3.9. Detection in real sample
The binding efficiency of CDs@amikacin with series of different concentrations of the bacterial E. coli was obtained from three different fruit and compared using florescence spectra. The fluorescence intensity of E. coli labeled with CDs@amikacin-1 linearly increases with the increasing concentration of bacterial cell of E. coli obtained from apple [Fig. 5(a and b)], orange [Fig. 5(c and d)], and pineapple [Fig. 5(e and f)] juice showed linear relations of fluorescence intensity with a limit of detection (LOD; at an S/N ratio of 3) approximately 340, 335, 376 cfu per mL respectively. The minimum detectable concentration of E. coli in these three samples was determined approximately 7.625 × 102 cfu per mL. The results suggest that our CDs@amikacin-1 probe has great potential for use in the rapid screening of E. coli in real samples.4. Conclusion
We here by constructed a facile method to synthesize amikacin conjugated CDs for selective labeling and detection of E. coli. It is found that the amikacin conjugated CDs efficiently detect the bacterial cell of E. coli with a noble detection limit of 552 cfu per mL. Our work provides a new option for the fabrication of bacteria binding drug functionalized CDs, which would also be significantly useful for the selective detection of pathogenic bacteria cell of E. coli in the real samples. In addition, the as synthesised amikacin conjugated CDs would be highly useful in many applications of biological field owing to its ultra-high photo stability with respect to the ionic strength and pH of the medium.Acknowledgements
This work was financially supported by the DST, Government of India (SB/FT/CS-068/2013) and Indian School of Mines, Dhanbad.References
- J. Wang and S.-T. Yau, Anal. Methods, 2014, 6, 5387–5391 RSC.
- P. A. Mosier-Boss, K. C. Sorensen, R. D. George and A. Obraztsova, Spectrochim. Acta, Part A, 2016, 153, 591–598 CrossRef CAS PubMed.
- L. Yang and Y. Li, Analyst, 2006, 131, 394–401 RSC.
- B. Mukhopadhyay, M. B. Martins, R. Karamanska, D. A. Russell and R. A. Field, Tetrahedron Lett., 2009, 50, 886–889 CrossRef CAS.
- Z. Chen, L. Cai, M. Chen, Y. Lin, D. Pang and H. Tang, Biosens. Bioelectron., 2015, 66, 95–102 CrossRef CAS PubMed.
- Y. Si, C. Grazon, G. Clavier, J. Rieger, J. Audibert, B. Sclavi and R. Méallet-Renault, Biosens. Bioelectron., 2016, 75, 320–327 CrossRef CAS PubMed.
- P. Luo, S. Sahu, S. Yang, S. Sonkar, J. Wang, H. Wang, G. LeCroy, L. Cao and Y. Sun, J. Mater. Chem. B, 2013, 1, 2116–2127 RSC.
- N. Gogoi and D. Chowdhury, J. Mater. Chem. B, 2014, 2, 4089–4099 RSC.
- V. Biju, Chem. Soc. Rev., 2014, 43, 744–764 RSC.
- T. Araújo, H. Oliveira, J. Telesa, J. Fabris, L. Oliveira and J. Mesquita, Appl. Catal., B, 2016, 182, 204–212 CrossRef.
- J. Jana, M. Ganguly, B. Das, S. Dhara, Y. Negishi and T. Pal, Talanta, 2016, 150, 253–264 CrossRef CAS PubMed.
- S. Chandra, S. Mitra, D. Laha, S. Bag, P. Das, A. Goswami and P. Pramanik, Chem. Commun., 2011, 47, 8587–8589 RSC.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113(43), 18546–18551 CAS.
- H. Li, F. Shao, H. Huang, J. Feng and A. Wang, Sens. Actuators, B, 2016, 226, 506–511 CrossRef CAS.
- H. Huang, C. Li, S. Zhu, H. Wang, C. Chen, Z. Wang, T. Bai, Z. Shi and S. Feng, Langmuir, 2014, 30(45), 13542–13548 CrossRef CAS PubMed.
- L. Cheng, Y. Li, X. Zhai, B. Xu, Z. Cao and W. Liu, ACS Appl. Mater. Interfaces, 2014, 6(22), 20487–20497 CAS.
- J. Chen, Y. Li, K. Lva, W. Zhong, H. Wang, Z. Wu, P. Yia and J. Jiang, Sens. Actuators, B, 2016, 224, 298–306 CrossRef CAS.
- A. Bourlinos, R. Zbořil, J. Petr and A. Bakandritsos, Chem. Mater., 2012, 24(1), 6–8 CrossRef CAS.
- C. Weng, H. Chang, C. Lin, Y. Shen, B. Unnikrishnan, Y. Li and C. Huang, Biosens. Bioelectron., 2015, 68, 1–6 CrossRef CAS PubMed.
- D. Zhong, Y. Zhuo, Y. Feng and X. Yang, Biosens. Bioelectron., 2015, 74, 546–553 CrossRef CAS PubMed.
- S. Nandi, M. Ritenberg and R. Jelinek, Analyst, 2015, 140, 4232–4237 RSC.
- B. Bhushan, S. U. Kumar and P. Gopinath, J. Mater. Chem. B, 2016, 4, 4862–4871 RSC.
- T. Ipekci, D. Seyman, H. Berk and O. Celik, J. Infect. Chemother., 2014, 20, 762–767 CrossRef CAS PubMed.
- E. Douka, E. Perivolioti, E. Kraniotaki, K. Fountoulis, F. Economidou, A. Tsakris, A. Skoutelis and C. Routsi, Int. J. Antimicrob. Agents, 2015, 45, 533–536 CrossRef CAS PubMed.
- M. R. Jacobs, S. Bajaksouzian, C. E. Good, M. M. Butler, J. D. Williams, N. P. Peet, T. L. Bowlin, A. Endimiani and R. A. Bonomo, Diagn. Microbiol. Infect. Dis., 2011, 69, 114–116 CrossRef CAS PubMed.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- C. H. Gayathri, P. Mayuri, K. Sankaran and A. S. Kumar, Biosens. Bioelectron., 2016, 82, 71–77 CrossRef CAS PubMed.
- L. Wen-Jie, Z. Xin-Ai, L. Chang-Feng, S. Jian-Zhong, J. Yu-Xiang and H. Chen-Yong, Chin. J. Anal. Chem., 2016, 44(7), 1015–1021 Search PubMed.
- N. S. K. Gunda, R. Chavali and S. K. Mitra, Analyst, 2016, 141, 2920–2929 RSC.
- Z. Yang, M. Xu, Y. Liu, F. He, F. Gao, Y. Su, H. Wei and Y. Zhang, Nanoscale, 2014, 6, 1890–1895 RSC.
- X. Teng, C. Ma, C. Ge, M. Yan, J. Yang, Y. Zhang, P. C. Morais and H. Bi, J. Mater. Chem. B, 2014, 2, 4631–4639 RSC.
- Y. Guo, Z. Wang, H. Shao and X. Jiang, Carbon, 2013, 583–589 CrossRef CAS.
- X. Shao, H. Gu, Z. Wang, X. Chai, Y. Tian and G. Shi, Anal. Chem., 2013, 85, 418–425 CrossRef CAS PubMed.
- S. Chandra, D. Laha, A. Pramanik, A. R. Chowdhuri, P. Karmakar and S. K. Sahu, Luminescence, 2016, 31, 81–87 CrossRef CAS PubMed.
- S. Chandra, A. R. Chowdhuri, T. K. Mahto and S. K. Sahu, Nanosci. Nanotechnol., 2016, 16, 1–9 CrossRef.
- S. Mohapatra, S. Sahu, N. Sinha and S. K. Bhutia, Analyst, 2015, 140, 1221–1228 RSC.
- R. Zhang and W. Chen, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS PubMed.
- Z. Tian, J. Xu, Y. Wang and G. Luo, Chem. Eng. J., 2016, 285, 20–26 CrossRef CAS.
- Y. Hao, Z. Gan, J. Xu, X. Wu and P. K. Chu, Appl. Surf. Sci., 2014, 311, 490–497 CrossRef CAS.
- B. Hana, W. Wang, H. Wu, F. Fang, N. Wang, X. Zhang and S. Xu, Colloids Surf., B, 2012, 100, 209–214 CrossRef PubMed.
- B. De and N. Karak, RSC Adv., 2013, 3, 8286–8290 RSC.
- J. R. Cox, G. A. McKay, G. D. Wright and E. H. Serpersu, J. Am. Chem. Soc., 1996, 118, 1295–1301 CrossRef CAS.
- M. Xu, R. Wang and Y. Li, Talanta, 2016, 148, 200–208 CrossRef CAS PubMed.
- S. Bera, G. G. Zhanel and F. Schweizer, J. Med. Chem., 2010, 53, 3626–3631 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra of amikacin; CDs and CDs@amikacin-1, plot of integrated fluorescence intensity versus UV-absorbance of the CDs@amikacin-1, CDs and quinine sulfate, effect of pH, ionic strength, organic molecules and irradiation time on the fluorescence intensity of CDs@amikacin-1 and CDs, the zeta potential study of CDs@amikacin-1 and CDs, FESEM image of the CDs@amikacin-1 and CDs, EDX spectra and elemental composition study of the synthesised CDs and CDs@amikacin-1, fluorescent images of E. coli labeled with CDs@amikacin-1 and CDs@amikacin-2. See DOI: 10.1039/c6ra15778e |
| This journal is © The Royal Society of Chemistry 2016 |