Antiepileptic drugs affect lipid oxidative markers- neuroprostanes and F2-dihomo-isoprostanes- in patients with epilepsy: differences among first-, second-, and third-generation drugs by UHPLC-QqQ-MS/MS
Sonia Medinaa,
Rubén Carrasco-Torresb,
Ma Isabel Amora,
Camille Ogerc,
Jean-Marie Galanoc,
Thierry Durandc,
Irene Villegas-Martínezb,
Stephane Auvind,
Federico Ferreresa and
Ángel Gil-Izquierdo*a
aResearch Group on Quality, Safety and Bioactivity of Plant Foods, Department of Food Science and Technology, CEBAS (CSIC), P.O. Box 164, Campus University Espinardo, 30100, Murcia, Spain. E-mail: angelgil@cebas.csic.es; Fax: +34 968396213; Tel: +34 968396363
bEpilepsy Unit, University Hospital Virgen de la Arrixaca, Murcia, Spain
cInstitut des Biomolécules Max Mousseron (IBMM), UMR 5247 – CNRS – University of Montpellier – ENSCM, Faculty of Pharmacy, Montpellier, France
dDepartment of Neuropediatric, Robert Debré Hospital, APHP, Paris, France
First published on 26th August 2016
Abstract
Metabolites of the non-enzymatic lipid peroxidation of polyunsaturated fatty acids (DHA, n-6DPA, and AdA), neuroprostanes (NeuroPs), and F2-dihomo-isoprostanes (F2-dihomo-IsoPs) have become important biomarkers for oxidative stress (OS) in neurodegenerative diseases, particularly in epilepsy. These biomarkers were measured by UHPLC-QqQ-MS/MS in the urine of 30 epileptic patients treated with different antiepileptic drugs (AEDs) (old treatment (n = 15) or new-generation treatments (n = 15)), in comparison with 15 healthy controls. After treatment with new-generation AEDs (levetiracetam, eslicarbazepine acetate, lacosamide, and zonisamide) a decrease was observed in the total urinary neuronal membrane degradation markers (NeuroPs) and neuromotor system degradation markers (F2-Dihomo-IsoPs), in comparison with the old treatment (carbamazepine, phenytoin, valproic acid) and control (no pharmacological treatment) groups. These results suggest that new-generation AEDs reduce the total NeuroPs/F2-Dihomo-IsoPs to levels similar to those of the control group and hence play an important role in antioxidant systems in epileptic patients. Unfortunately, little is known currently about the neuroprotective effect of second- and third-generation AEDs; so, more clinical studies need to be performed to clarify the relationships among chronic treatment with newer AEDs, oxidative stress, and epilepsy.
1. Introduction
Epilepsy is one of the most common and diverse neurological disorders and it is defined by a state of recurrent, spontaneous seizures, which can be convulsive or non-convulsive episodes.1 It has been suggested that oxidative damage and neuronal cell death are common pathological processes that can contribute to epileptogenesis.2 It has been reported that the increased generation of free radicals can cause some forms of epilepsy, and also increase the risk of seizures recurrence.3 Antiepileptic drugs (AEDs) are widely used in the treatment of epilepsy, as well as in various other neurological and psychiatric disorders.4 The AEDs are considered the first-line drugs (carbamazepine (CBZ), phenobarbital (PB), and valproic acid (VPA)) which increase free radical formation; this causes oxidative damage within neuronal cells, increases the peroxidation of neuronal membrane lipids, and reduces the protective effects of antioxidants.5 Due to this, during the last three decades, 14 new AEDs have been licensed for clinical use. They can be divided into new second-generation (felbamate (FBM), gabapentin (GBP), lamotrigine (LTG), levetiracetam (LEV), oxcarbazepine (OXC), pregabalin (PGB), rufinamide (RFN), stiripentol (STP), tiagabine (TGB), topiramate (TPM), vigabatrin (VGB), and zonisamide (ZNS)) and third-generation (eslicarbazepine acetate (ESL) and lacosamide (LCM)) AEDs. Compared with first-generation AEDs, second- and third-generation AEDs interact less (pharmacokinetics and pharmacodynamics), and so lead to less complicated therapeutic outcomes and fewer complications for patients.4In recent years, important advances have been made in the diagnosis of epilepsy. However, the understanding of the molecular mechanisms underlying epileptogenesis is still incomplete. Among various factors supposed to play a role in this disorder, the role of oxidative stress (OS) in neurological diseases has recently emerged.6 Oxidative stress is a biochemical state in which reactive oxygen species (ROS) are generated and it has been associated with pathological states including epilepsy; therefore, oxidative injury (OI) may play a crucial role in the initiation and progression of this disease.7 Therein, neuroprostanes (NeuroPs) and F2-dihomo-isoprostanes (F2-dihomo-IsoPs) – a series of compounds formed non-enzymatically through free radical induced peroxidation of DHA, n-6DPA, and AdA, respectively – are implicated in the pathophysiological status of epilepsy.8
The aim of this study was to evaluate the levels of urinary NeuroPs/F2-dihomo-IsoPs in epileptic patients, and then to determine whether treatment with first-(classic treatment) or second/third-generation (new-generation treatment) AEDs influences the urinary NeuroPs/F2-dihomo-IsoPs levels.
2. Materials and methods
2.1 Chemicals and reagents
Nine NeuroPs – 4(RS)-F4t-NeuroP; d4-4(RS)-F4t-NeuroP; 10-epi-10-F4t-NeuroP; d4-10-epi-10-F4t-NeuroP; 10-F4t-NeuroP; d4-10-F4t-NeuroP; 4-F4t-NeuroP; 4-epi-4-F3t-NeuroP; and 4-F3t-NeuroP – as well as four F2-dihomo-IsoPs – Ent-7(RS)-7-F2t-dihomo-IsoP; Ent-7(S)-7-F2t-dihomo-IsoP; 17-F2t-dihomo-IsoP; and 17-epi-17-F2t-dihomo-IsoP – were synthesized according to our published procedures.9–12 The β-glucuronidase, type H2, from Helix pomatia, and BIS-TRIS (bis-(2-hydroxyethyl)-amino-tris(hydroxymethyl)-methane) used in this study were from Sigma-Aldrich (St. Louis, MO, USA). All LC-MS grade solvents were from J.T. Baker (Phillipsburg, NJ, USA). Chlorhydric acid was purchased from Panreac (Castellar del Vallés, Barcelona, Spain), and the Strata X-AW (100 mg 3 mL−1) SPE cartridges from Phenomenex (Torrance, CA, USA).2.2 Selection of study participants
Thirty ambulatory epileptic patients from the Epilepsy Unit of the Virgen de la Arrixaca Hospital (Murcia, Spain) were enrolled in this study. Based on the type of AED treatment, the patients were divided into two groups, 15 patients receiving first-generation AEDs (classic treatment (CE)) and 15 receiving second- or third-generation AEDs (new-generation treatment (NGE)). All volunteers with epilepsy were seizure-free and without any changes in the daily dose of monotherapy AEDs for the last six months prior to their inclusion. All these patients had been diagnosed with epilepsy, based on clinical criteria supported by electroencephalographic recordings and cerebral magnetic resonance imaging (MRI). Standard EEG was performed at seizure onset and, if it was not conclusive, video-EEG monitoring was used. A cerebral MRI scan (1.5T or 3T when available) was performed in every patient in order to detect any structural lesion, such as cortical dysplasia, hippocampal sclerosis, brain tumors, or vascular lesions. Epilepsy was then classified, according to the International League Against Epilepsy (ILAE) criteria, as genetic, structural, or of unknown origin. The physical characteristics and clinical profiles of the subjects are summarized in Table 1. The control subjects (C; n = 15) were age-matched individuals without clinical evidence of epilepsy or other neurological diseases, being otherwise healthy subjects. Importantly, all individuals in this study were non-smokers and had no dietary restrictions; during the study, the women were not menstruating. The control group did not receive any medication or drug intake (we noted the specific absence of the acute administration of anti-inflammation drugs). All patients gave written informed consent for the experiment. This study was approved by the Bioethics Committee of the Virgen de la Arrixaca Hospital (EPA-SP (IRE-CBZ-2014-01)) and the research was carried out in compliance with the Declaration of Helsinki for all human experimental investigations.| Control group (n = 15) | Epileptic CE (n = 15) | Epileptic NGE (n = 15) | |
|---|---|---|---|
| a Data are presented as mean ± SD. Abbreviations: BMI (Body Mass Index); AED (Antiepileptic Drug); CBZ (Carbamazepine); ESL (Eslicarbazepine acetate); GGT (Gamma Glutamyl Transferase); GOT (Glutamic Oxaloacetic Transaminase); GPT (Glutamic Pyruvate Transaminase); LCM (Lacosamide); LEV (Levetiracetam); PHT (Phenytoin); TSH (Thyroid Stimulating hormone); FT4 (Free Thyroxine); VPA (Valproic Acid); ZNS (Zonisamide). No significant differences either between the control and AEDs treated groups or between the two treated (CE and NGE) groups were found. | |||
| Age (years) | 32 ± 8 | 38.5 ± 11.8 | 36.1 ± 17.7 |
| Gender male (n) | 10 | 12 | 11 |
| BMI (kg m−2) | 22.5 ± 3.2 | 27.4 ± 4.7 | 24.5 ± 4.8 |
| Epilepsy type (n) | |||
| • Genetic | — | 3 | 2 |
| • Structural | — | 8 | 7 |
| • Unknown origin | — | 4 | 6 |
| Seizure type (n) | |||
| • Generalized | — | 5 | 7 |
| • Focal | — | 5 | 4 |
| • Evolving to a bilateral convulsive seizures | — | 5 | 4 |
| Duration of epilepsy (years) | — | 12.9 ± 8.1 | 7.3 ± 6.9 |
| Duration of therapy (months) | — | 104.9 ± 85.8 | 34.3 ± 22.9 |
| AED (n (mean dose mg per d)) | |||
| • CBZ | — | 6 (629) | — |
| • PHT | — | 1 (300) | — |
| • VPA | — | 8 (1169) | — |
| • LEV | — | — | 11 (1325) |
| • LCM | — | — | 1 (200) |
| • ESL | — | — | 2 (1000) |
| • ZNS | — | — | 1 (300) |
| Calcium (mg dL−1) | 9.9 ± 0.4 | 9.6 ± 0.5 | 9.7 ± 0.2 |
| Sodium (mEq L−1) | 134.8 ± 16.9 | 141.2 ± 2.2 | 141.1 ± 2.5 |
| Glucose (mg dL−1) | 87.5 ± 6.9 | 98.1 ± 29.5 | 91.1 ± 6.7 |
| Urea (mg dL−1) | 32.5 ± 3.7 | 34.1 ± 10 | 31.0 ± 9.6 |
| Creatinine (mg dL−1) | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| Bilirubin total (mg dL−1) | 0.7 ± 0.2 | 0.4 ± 0.2 | 0.8 ± 0.3 |
| GOT (U L−1) | 26.0 ± 6.1 | 21.3 ± 7.8 | 22.7 ± 12.2 |
| GPT (U L−1) | 24.0 ± 8.4 | 22.6 ± 8.6 | 22.7 ± 12.5 |
| GGT (U L−1) | 14.8 ± 3.8 | 33.2 ± 20.2 | 20.7 ± 13.2 |
| FT4 (ng dL−1) | 1.2 ± 0.1 | 1.1 ± 0.1 | 2.2 ± 0.7 |
| TSH (uUI mL−1) | 2.1 ± 0.7 | 2.5 ± 1.6 | 1.1 ± 0.1 |
2.3 Standard and sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to obtain the appropriate working solutions containing 10 analytes at a concentration of 1000 nmol L−1. For the determination of the calibration curves, 12 successive dilutions were prepared. All solutions were stored at −80 °C. The NeuroPs/F2-dihomo-IsoPs concentrations were calculated from standard curves freshly prepared each day.
1, v/v) to obtain the appropriate working solutions containing 10 analytes at a concentration of 1000 nmol L−1. For the determination of the calibration curves, 12 successive dilutions were prepared. All solutions were stored at −80 °C. The NeuroPs/F2-dihomo-IsoPs concentrations were calculated from standard curves freshly prepared each day.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g during 5 minutes. Briefly, the urine samples were applied to Strata X-AW SPE cartridges from Phenomenex (Torrance, CA, USA) previously conditioned and equilibrated and washed with 4 mL of water. The analytes were eluted with 1 mL of MeOH and dried using a SpeedVac concentrator (Savant SPD121P, Thermo Scientific, MA, USA). The extracts were reconstituted with 200 μL of solvent A/B (90
000 g during 5 minutes. Briefly, the urine samples were applied to Strata X-AW SPE cartridges from Phenomenex (Torrance, CA, USA) previously conditioned and equilibrated and washed with 4 mL of water. The analytes were eluted with 1 mL of MeOH and dried using a SpeedVac concentrator (Savant SPD121P, Thermo Scientific, MA, USA). The extracts were reconstituted with 200 μL of solvent A/B (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) and filtered through a 0.45 μm filter of PVDF (Millipore, MA, USA). Then, 20 μL were analyzed in a UHPLC-QqQ-MS/MS.
10, v/v) and filtered through a 0.45 μm filter of PVDF (Millipore, MA, USA). Then, 20 μL were analyzed in a UHPLC-QqQ-MS/MS.2.4 UHPLC-QqQ-MS/MS analyses
The separation and quantification of the NeuroPs and F2-dihomo-IsoPs in the urine were performed using a UHPLC coupled with a 6460 QqQ-MS/MS (Agilent Technologies, Waldbronn, Germany), and the analytical method previously described.8 Briefly, chromatographic separation was carried out on an ACQUITY BEH C18 column (2.1 × 50 mm, 1.7 μm pore size) (Waters, MA, USA). The mass spectrometry analysis was performed in the multiple reaction monitoring (MRM) mode, in the negative ionization mode. The mobiles phases were solvent A (Milli-Q water/acetic acid, 99.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, v/v) and solvent B (methanol/acetic acid, 99.99
0.01, v/v) and solvent B (methanol/acetic acid, 99.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, v/v). The flow rate was 0.2 mL min−1. The ESI conditions and ion optics were those previously described.8 Data acquisition and processing were performed using MassHunter software version B.04.00 (Agilent Technologies, Walbronn, Germany). The quantitative analysis of NeuroPs/F2-dihomo-IsoPs was performed using the authentic markers synthesized by Durand's team.
0.01, v/v). The flow rate was 0.2 mL min−1. The ESI conditions and ion optics were those previously described.8 Data acquisition and processing were performed using MassHunter software version B.04.00 (Agilent Technologies, Walbronn, Germany). The quantitative analysis of NeuroPs/F2-dihomo-IsoPs was performed using the authentic markers synthesized by Durand's team.
2.5 Statistical analyses
The quantitative data are presented as mean ± SD (standard deviation). Specific differences in the amounts of NeuroPs and F2-dihomo-IsoPs excreted were calculated as ng per 24 h urine. The samples of the control and AEDs treatment groups were examined by one-way ANOVA (three groups: control, classic AEDs treatment, and new-generation AEDs treatment) followed by Tukey's HSD (Honestly Significant Difference) test. Statistical analyses were performed using the SPSS 21.0 software package (LEAD Technologies Inc., Chicago, USA) and the level of statistical significance was set at P < 0.05.3. Results
3.1 Characteristics of the study population
The study was performed in 30 patients diagnosed with epilepsy and 15 healthy volunteers (control group). The epileptic patients were divided into two groups of 15, depending on the AED received: classic and new-generation AEDs (Table 1). The mean age of the patients receiving classic AEDs was 38.5 ± 11.8 years, vs. 36.1 ± 17.7 years for patients on new-generation AEDs. Of the patients treated with classic AEDs, 53% had structural epilepsy and 26% had epilepsy of unknown origin, while 47% of those on newer AEDs were diagnosed with structural epilepsy and 40% had epilepsy of unknown origin. This classification was carried out under guidelines previously published by Berg and colleagues.13 The duration of the epilepsy and therapy was greater in the classic AEDs group than with new-generation drugs (12.9 ± 8.1 years vs. 7.3 ± 6.9 years and 104.9 ± 85.8 months vs. 34.3 ± 22.9 months, respectively). Biochemical parameters are shown in Table 1.3.2 Qualitative profile of neuroprostanes and F2-dihomo-isoprostanes in the epileptic subject urines
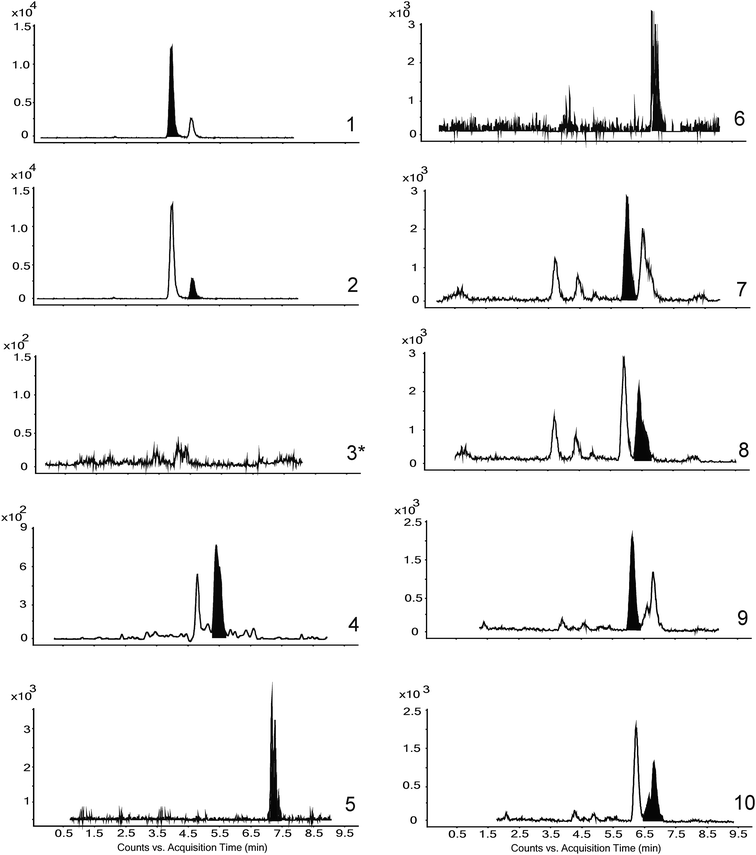
The analysis of NeuroPs/F2-dihomo-IsoPs allowed the detection of nine lipid oxidation markers. The separation of the compounds – using the method developed by Medina and colleagues8-was satisfactory (Fig. 1). The only NeuroP not detected was 4(S)-F4t-NeuroP. Moreover, for the unequivocal identification of the target compounds, the most intensive MRM transition was selected (see Fig. 1 and its footnote). Some MRM transitions were not exclusive to a single compound; so, some compounds were differentiated by their distinct retention times.3.3 Effect of classic and new-generation antiepileptic drugs on urinary levels of lipid oxidation markers
The method of urine analysis developed by Medina and colleagues8 allowed the quantification of nine lipid oxidation markers (five NeuroPs and four F2-dihomo-IsoPs) in subjects with epilepsy treated with different AEDs, classic (first-generation) and new generation (second- and third-generation), compared with the control group. There was a statistically significant increase (P < 0.05) in the subtotal of neuronal membrane degradation markers (NeuroPs) in the epileptic patients treated with classic AEDs (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 565.7 ± 9306.9 ng 24 h−1), when compared with the new-generation AEDs treatment patients (15
565.7 ± 9306.9 ng 24 h−1), when compared with the new-generation AEDs treatment patients (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070.1 ± 3398.1 ng 24 h−1) and the control group (13
070.1 ± 3398.1 ng 24 h−1) and the control group (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 218.6 ± 4683.2 ng 24 h−1). Similarly, the subtotal of F2-dihomo-isoprostanes (neuromotor system degradation markers) was higher in patients receiving classic-generation drugs (31
218.6 ± 4683.2 ng 24 h−1). Similarly, the subtotal of F2-dihomo-isoprostanes (neuromotor system degradation markers) was higher in patients receiving classic-generation drugs (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224.9 ± 7497.1 ng 24 h−1) than in epileptic subjects treated with new-generation AEDs (20
224.9 ± 7497.1 ng 24 h−1) than in epileptic subjects treated with new-generation AEDs (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 ± 4968.7 ng 24 h−1) or the control group (24
441 ± 4968.7 ng 24 h−1) or the control group (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505.6 ± 6025 ng 24 h−1).
505.6 ± 6025 ng 24 h−1).
Regarding the neuronal membrane degradation markers, two (4-epi-4-F3t-NeuroP and 10-epi-10-F4t-NeuroP) did not show statistically significant differences among the groups. Moreover, one compound (4(R)-F4t-NeuroP) was only detected and quantified in the group of patients treated with first-generation AEDs.
The data show a decrease in four lipid oxidation markers (4-F3t-NeuroP, 17-F2t-Dihomo-IsoP, Ent-7(R)-7-F2t-dihomo-IsoP, and Ent-7(S)-7-F2t-dihomo-IsoP) when the patients had received new-generation AEDs, compared with the classic treatment and control group (see Fig. 2). So, 4-F3t-NeuroP decreased by ∼56% and ∼37% (new-generation treatment and control, respectively) relative to the classic treatment. Also, the most marked reduction (∼78%) was observed for 17-F2t-dihomo-IsoP, when compared with the classic treatment and new generation treatment groups. In the same sense, the amounts of Ent-7(R)-7-F2t-Dihomo-IsoP and Ent-7(S)-7-F2t-dihomo-IsoP decreased by ∼43% and ∼21%, respectively, comparing the new-generation treatment with the first-generation AEDs. Moreover, only two compounds (10-F4t-NeuroP and 17-epi-17-F2t-dihomo-IsoP) showed increases in the patients treated with new-generation drugs (rises of ∼62% and ∼26%, respectively), compared to the classical medication (Fig. 2). A very important fact about this study is that 11 of the 15 patients in the new AEDs group were on LEV (a drug with little interaction in the liver). However, the oxidative marker levels after treatment with other new AEDs (ESL, LCM, or ZNS) were statistically similar to those in the volunteers receiving LEV alone. In the same sense, in the volunteers treated with classic AEDs, the NeuroPs/F2-dihomo-IsoPs levels showed no statistical differences among the CBZ, PHT, or VPA treatments.
4. Discussion
Lipid peroxidation is one of the most biologically important free radical reactions and it has been pointed out as a key chemical event in the OS associated with several inborn and acquired pathologies.14 The most widely used test for OS is measurement of malondialdehyde (MDA), a product of lipid peroxidation in a thiobarbituric acid-reacting substances (TBARS) assay. However, the use of this analysis to evaluate the OS status is problematic because MDA is not a specific product of lipid peroxidation and the TBARS assay is not specific for MDA.15,16 The recently developed measurement of F2-IsoPs, NeuroPs, and F2-dihomo-IsoPs is the best available assay of lipid peroxidation in neurodegenerative diseases.8,17,18In all analyses, one very important aspect is the technique used and its limitations. The GC/MS device was the one used first for the quantification of NeuroPs/F2-dihomo-IsoPs.19 However, due to its handicaps (extensive sample preparation, weak specificity, and an inability to isolate isomers of compounds), other devices such as LC/MS-MS are gaining more attention.20 As well as LC/MS, other techniques, such as enzyme-linked immunosorbert assay (ELISA) and radioimmunoassay (RIA), were developed for the determination of IsoPs (8-iso-PGF2α) in the urine of epileptic patients21,22 and for the detection of NeuroPs/F2-dihomo-IsoPs.23 So, immunological methods were chosen because these techniques are reproducible and easy to perform. However, the cross-reactivity among the different NeuroPs/F2-dihomo-IsoPs and their demonstrated over-estimation constituted significant disadvantages compared to LC/MS-MS, making them inappropriate for clinical application.24 Also, LC/MS (specifically UHPLC) is superior to other techniques (such as GC/NICI-MS and ELISA/RIA) in the identification of different regioisomers and diasteroisomers of NeuroPs/F2-dihomo-IsoPs;8 moreover, this technique is much faster.
From a clinical point of view, previous studies reported that F4-NeuroPs were increased in patients with neurological diseases (such as aneurysmal subarachnoid hemorrhage, Rett syndrome, Alzheimer's disease, or atherosclerosis), compared to healthy control groups.25–28 Indeed, in comparison with these reports, our study has detected differences among groups (C, CE, and NGE) and has quantified a greater amount of NeuroPs/F2-dihomo-IsoPs. This may be due to the greater suitability and selectivity of the UHPLC-MS technique applied in our study. As well, the enzymatic hydrolysis step was crucial for good and real quantification in the urine samples. A recently published study showed that these compounds are excreted as glucuronide conjugates; so, the hydrolysis of the urine samples could allow measurement of greater amounts of NeuroPs/F2-dihomo-IsoPs, compared with non-hydrolyzed samples.8
In recent years, important advances have been made in the diagnosis and treatment of neurological diseases in general and of epilepsy in particular. It has been shown that, apart from other known mechanisms in epileptogenesis, OS and generation of ROS are implicated in seizure disorders.21 Similarly, increased levels of OS markers have been found in different experimental models of epilepsy.29 So, lipid peroxidation markers (8-iso-PGF2α, also named 15-F2t-IsoP) were found to have increased in abundance in rats after VPA (classical AEDs) treatment.30 In the same sense, in humans, Michoulas and colleagues showed increased urinary levels of 8-iso-PGF2α in epileptic children who were treated with VPA. This may indicate that VPA induces the OS in epileptic patients;31 it indicates also that long-term use of AEDs may affect lipid oxidative damage and antioxidant defense in epileptic patients.32 In our study, there was a difference in the treatment duration between the two groups (classic and new-generation AEDs); however, the effect of the AEDs treatment duration on the generation of OS markers is unclear. Chuang and colleagues reported that long-term monotherapy with diverse AEDs had no effect on lipid peroxidation markers (TBARS) or on inflammation markers such as hs-CRP.33
Regarding treatment with new-generation AEDs, only one study has reported LEV-induced formation of 8-iso-PGF2α in epileptic patients.22 Several previous reports have studied the properties of different AEDs (VPA, CBZ, LEV, and ZNS) and their behavior in the diverse pathways of pro-oxidation/antioxidation. A problem that arises when trying to clarify the role of AEDs in this field is the heterogeneity in the samples studied: for example, blood or urine of epileptic patients,34 rat astrocytes cultures,35 plasma or tissues of rat,36 or rodent hippocampus.37 In a similar vein, there were also differences among studies performed in vitro and in vivo experiments, in most cases involving the same AEDs.38
The relationship between OS and AEDs is documented in the scientific literature in a poor and controversial way, especially regarding the differences between classic and new-generation drugs. The classic drugs such as VPA or CBZ could induce overproduction of ROS, whereas new-generation drugs could have a “scavenger” function in relation to ROS production in human and animal models.39 So, VPA seems to have pro-oxidant activity: Martinez-Ballesteros and colleagues reported that plasma samples of patients treated with this AED showed vulnerability to OS that was directly proportional to the plasma drug concentration.40 On the other hand, previous studies showed that AEDs did not influence the oxidative markers, suggesting the presence of seizure-induced OS.41 Besides, findings concerning the effects of AEDs and OS indicated that the pro-oxidant/antioxidant balance of epileptic patients was modified by AEDs therapy.42 Therefore, knowledge of how these drugs could modulate this oxidative system will open a new therapeutic window for epilepsy treatments in which OS is one of the underlying mechanisms.43 Indeed, in the report of French and colleagues,44 it is cited that it is impossible to confirm that “all old AEDs are bad” or “all new AEDs are good”. However, the absence of hepatic enzyme induction/inhibition when most of the newer AEDs are used provides one more advantage.44 So, this study provides mechanistic support for further research in this field. The first aspect that must be investigated is the true impacts of AEDs on the oxidative status of epileptic patients, being as several different factors, including pharmacogenetics, contribute to inter-individual variability in drug response.
5. Conclusion
The present findings show that treatment with new-generation AEDs globally reduces the excretion of NeuroPs/F2-dihomo-IsoPs to values similar to those in the control group, indicating a positive effect of these AEDs on the antioxidant status of epileptic patients. Unfortunately, little is known about the neuroprotective effect of second- and third-generation AEDs, so more investigation needs to be done in the clinical field to clarify the relationships among chronic treatment of AEDs, oxidative stress, and epilepsy. So, it would be of interest to conduct additional prospective studies specifically designed to clarify the effect of pharmacological treatment in patients with epilepsy.Abbreviations
| AdA | Adrenic acid |
| AEDs | Antiepileptic drugs |
| CBZ | Carbamazepine |
| DHA | Docosahexaenoic acid |
| Dihomo-IsoPs | Dihomo-isoprostanes |
| DPA | Docosapentanoic acid |
| EEG | Electroencephalography |
| ELISA | Enzyme-linked immunosorbert assay |
| ESI | Electrospray ionization |
| ESL | Eslicarbazepine acetate |
| FBM | Felbamate |
| GBP | Gabapentin |
| GC-NICI-MS | Gas chromatography-negative ion chemical ionization-mass spectrometry |
| hs-CRP | High-sensitivity C-reactive protein |
| ILAE | International league against epilepsy |
| LC/MS-MS | Liquid chromatography tandem mass spectrometry |
| LCM | Lacosamide |
| LC-MS | Liquid chromatography mass spectrometry |
| LEV | Levetiracetam |
| LTG | Lamotrigine |
| MDA | Malondialdehyde |
| MRI | Magnetic resonance imaging |
| MRM | Multiple reaction monitoring |
| NeuroPs | Neuroprostanes |
| OI | Oxidative injury |
| OS | Oxidative stress |
| OXC | Oxcarbazepine |
| PB | Phenobarbital |
| PGB | Pregabalin |
| RFN | Rufinamide |
| RIA | Radioimmunoassay |
| SPE | Solid phase extraction |
| STP | Stiripentol |
| TBARS | Thiobarbituric acid-reacting substances |
| TGB | Tiagabine |
| TPM | Topiramate |
| UHPLC-QqQ-MS/MS | Ultrahigh pressure liquid chromatography-triple quadrupole-tandem mass spectrometry |
| VGB | Vigabatrin |
| VPA | Valproic acid |
| ZNS | Zonisamide |
Acknowledgements
This work has been partially funded by the “Fundación Séneca de la Región de Murcia” Grupo de Excelencia 19900/GERM/15. We are grateful to Dr David Walker (native English speaker) for his reviews of the English grammar and style of the current report.References
- W. Löscher, H. Klitgaard, R. E. Twyman and D. Schmidt, Nat. Rev. Drug Discovery, 2013, 12, 757–776 CrossRef PubMed.
- Y. C. Chuang, Acta Neurol. Taiwan, 2010, 19, 3–15 Search PubMed.
- S. A. Hamed, M. M. Abdellah and N. El-Melegy, J. Pharmacol. Sci., 2004, 96, 465–473 CrossRef CAS.
- C. Johannessen Landmark, P. G. Larsson, E. Rytter and S. I. Johannessen, Epilepsy Res., 2009, 87, 31–39 CrossRef CAS PubMed.
- P. Maertens, P. Dyken, W. Graf, C. Pippenger, R. Chronister and A. Shah, Am. J. Med. Genet., 1995, 57, 225–228 CrossRef CAS PubMed.
- V. Shukla, S. K. Mishra and H. C. Pant, Adv. Pharmacol. Sci., 2011, 2011, 572634 Search PubMed.
- B. Martinc, I. Grabnar and T. Vovk, Curr. Neuropharmacol., 2012, 10(4), 328–343 CrossRef CAS PubMed.
- S. Medina, I. D. Miguel-Elizaga, C. Oger, J. M. Galano, T. Durand, M. Martinez-Villanueva, M. L. Castillo, I. Villegas-Martinez, F. Ferreres, P. Martinez-Hernandez and A. Gil-Izquierdo, Free Radical Biol. Med., 2015, 79, 154–163 CrossRef CAS PubMed.
- A.-L. Auvinet, B. Eignerová, A. Guy, M. Kotora and T. Durand, Tetrahedron Lett., 2009, 50, 1498–1500 CrossRef CAS.
- A. Guy, C. Oger, J. Heppekausen, C. Signorini, C. De Felice, A. Furstner, T. Durand and J. M. Galano, Chemistry, 2014, 20, 6374–6380 CrossRef CAS PubMed.
- C. Oger, Y. Brinkmann, S. Bouazzaoui, T. Durand and J. M. Galano, Org. Lett., 2008, 10, 5087–5090 CrossRef CAS PubMed.
- C. Oger, V. Bultel-Ponce, A. Guy, L. Balas, J. C. Rossi, T. Durand and J. M. Galano, Chemistry, 2010, 16, 13976–13980 CrossRef CAS PubMed.
- A. T. Berg, S. F. Berkovic, M. J. Brodie, J. Buchhalter, J. H. Cross, W. van Emde Boas, J. Engel, J. French, T. A. Glauser, G. W. Mathern, S. L. Moshe, D. Nordli, P. Plouin and I. E. Scheffer, Epilepsia, 2010, 51, 676–685 CrossRef PubMed.
- B. Halliwell and S. Chirico, Am. J. Clin. Nutr., 1993, 57, 724S–725S Search PubMed.
- E. Arhan, A. Serdaroglu, B. Ozturk, H. S. Ozturk, A. Ozcelik, N. Kurt, E. Kutsal and N. Sevinc, Seizure, 2011, 20, 138–142 CrossRef PubMed.
- P. M. Manmohan Krishna Pandey and P. K. Maheshwari, J. Clin. Diagn. Res., 2012, 6, 590–592 Search PubMed.
- T. Durand, C. De Felice, C. Signorini, C. Oger, V. Bultel-Poncé, A. Guy, J. M. Galano, S. Leoncini, L. Ciccoli, A. Pecorelli, G. Valacchi and J. Hayek, Biochimie, 2013, 95, 86–90 CrossRef CAS PubMed.
- E. Miller, A. Morel, L. Saso and J. Saluk, Oxid. Med. Cell. Longevity, 2014, 572491, 29 Search PubMed.
- L. J. Roberts Ii, T. J. Montine, W. R. Markesbery, A. R. Tapper, P. Hardy, S. Chemtob, W. D. Dettbarn and J. D. Morrow, J. Biol. Chem., 1998, 273, 13605–13612 CrossRef.
- C. Vigor, J. Bertrand-Michel, E. Pinot, C. Oger, J. Vercauteren, P. Le Faouder, J. M. Galano, J. C. Lee and T. Durand, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 964, 65–78 CrossRef CAS PubMed.
- M. Ercegovac, N. Jovic, T. Simic, L. Beslac-Bumbasirevic, D. Sokic, T. Djukic, A. Savic-Radojevic, M. Matic, J. Mimic-Oka and M. Pljesa-Ercegovac, Seizure, 2010, 19, 205–210 CrossRef PubMed.
- H. Ozden, S. C. Kabay, A. Toker, M. C. Ustüner, C. Ozbayer, D. Ustüner and H. V. Günes, Seizure, 2010, 19, 514–516 CrossRef PubMed.
- H. C. Yen, J. Biomed. Lab. Sci., 2010, 22, 1–11 Search PubMed.
- D. M. Callewaert and C. Sloan, Methods Mol. Biol., 2010, 610, 435–449 CAS.
- C. De Felice, C. Signorini, T. Durand, C. Oger, A. Guy, V. Bultel-Poncé, J. M. Galano, L. Ciccoli, S. Leoncini, M. D'Esposito, S. Filosa, A. Pecorelli, G. Valacchi and J. Hayek, J. Lipid Res., 2011, 52, 2287–2297 CrossRef CAS PubMed.
- C. Gladine, J. W. Newman, T. Durand, T. L. Pedersen, J. M. Galano, C. Demougeot, O. Berdeaux, E. Pujos-Guillot, A. Mazur and B. Comte, PLoS One, 2014, 9, e89393 Search PubMed.
- Y. P. Hsieh, C. L. Lin, A. L. Shiue, H. Yin, J. D. Morrow, J. C. Hsu, T. C. Hsieh, H. J. Wei and H. C. Yen, Free Radical Biol. Med., 2009, 47, 814–824 CrossRef CAS PubMed.
- C. Signorini, C. De Felice, S. Leoncini, A. Giardini, M. D'Esposito, S. Filosa, F. Della Ragione, M. Rossi, A. Pecorelli, G. Valacchi, L. Ciccoli and J. Hayek, Clin. Chim. Acta, 2011, 412, 1399–1406 CrossRef CAS PubMed.
- S. Grosso, M. Longini, A. Rodriguez, F. Proietti, B. Piccini, P. Balestri and G. Buonocore, J. Neurol. Sci., 2011, 300, 103–106 CrossRef CAS PubMed.
- V. Tong, X. W. Teng, S. Karagiozov, T. K. Chang and F. S. Abbott, Free Radical Biol. Med., 2005, 38, 1471–1483 CrossRef CAS PubMed.
- A. Michoulas, V. Tong, X. W. Teng, T. K. Chang, F. S. Abbott and K. Farrell, J. Pediatr., 2006, 149, 692–696 CrossRef CAS PubMed.
- M. M. ALshafei, World Appl. Sci. J., 2013, 28, 316–323 CAS.
- Y. C. Chuang, H. Y. Chuang, T. K. Lin, C. C. Chang, C. H. Lu, W. N. Chang, S. D. Chen, T. Y. Tan, C. R. Huang and S. H. Chan, Epilepsia, 2012, 53, 120–128 CrossRef CAS PubMed.
- A. O. Varoglu, A. Yildirim, R. Aygul, O. L. Gundogdu and Y. N. Sahin, Clin. Neuropharmacol., 2010, 33, 155–157 CrossRef CAS PubMed.
- M. N. Stienen, A. Haghikia, H. Dambach, J. Thone, M. Wiemann, R. Gold, A. Chan, R. Dermietzel, P. M. Faustmann, D. Hinkerohe and N. Prochnow, Br. J. Pharmacol., 2011, 162, 491–507 CrossRef CAS PubMed.
- V. Tong, T. K. Chang, J. Chen and F. S. Abbott, Free Radical Biol. Med., 2003, 34, 1435–1446 CrossRef CAS PubMed.
- A. A. Oliveira, J. P. Almeida, R. M. Freitas, V. S. Nascimento, L. M. Aguiar, H. V. Junior, F. N. Fonseca, G. S. Viana, F. C. Sousa and M. M. Fonteles, Cell. Mol. Neurobiol., 2007, 27, 395–406 CrossRef CAS PubMed.
- C. C. T. Aguiar, A. L. B. Almeida, P. V. P. Arajo, R. N. D. C. D. Abreu, E. M. C. Chaves, O. C. D. Vale, D. S. Macdo, D. J. Woods, M. M. Fonteles and S. M. M. Vasconcelos, Oxid. Med. Cell. Longevity, 2012, 795259 Search PubMed.
- M. Naziroglu and V. A. Yurekli, Cell. Mol. Neurobiol., 2013, 33, 589–599 CrossRef CAS PubMed.
- C. Martínez-Ballesteros, E. Pita-Calandre, Y. Sánchez-González, C. M. Rodríguez-López and A. Agil, Rev. Neurol., 2004, 38, 101–106 Search PubMed.
- B. Menon, K. Ramalingam and R. V. Kumar, Seizure, 2012, 21, 780–784 CrossRef PubMed.
- E. Solowiej and W. Sobaniec, Neurol. Neurochir. Pol., 2003, 37, 991–1003 Search PubMed.
- N. Cardenas-Rodriguez, B. Huerta-Gertrudis, L. Rivera-Espinosa, H. Montesinos-Correa, C. Bandala, L. Carmona-Aparicio and E. Coballase-Urrutia, Int. J. Mol. Sci., 2013, 14, 1455–1476 CrossRef PubMed.
- J. A. French and D. M. Gazzola, Ther. Adv. Drug Saf., 2011, 2(4), 141–158 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |