Biodegradable polymeric nanostructures in therapeutic applications: opportunities and challenges
Abstract
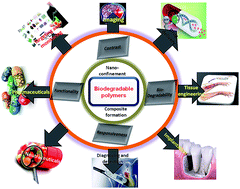
Biodegradable polymeric nanostructures (BPNs) have shown great promise in different therapeutic applications such as diagnosis, imaging, drug delivery, cosmetics, organ implants, and tissue engineering. BPNs exhibit superior properties over their respective bulk materials, overcoming the limitations of traditional polymers to address numerous important medical problems. Furthermore, the addition of other components can enable specific functionalities of the nanostructures to be modulated for tunable applications. This review emphasizes the state-of-the-art of biodegradable polymeric nanostructures, preparative methods, therapeutic uses, and challenging issues concerning the commercialization of emerging BPN-based therapeutics with respect to their future perspectives.

 Please wait while we load your content...
Please wait while we load your content...