Effect of fractions of kolaviron on some indices of benign prostatic hyperplasia in rats: identification of the constituents of the bioactive fraction using GC-MS
Kalu Winnera,
Okafor Polycarpa,
Ijeh Ifeomaa and
Eleazu Chinedumb
aDepartment of Biochemistry, Michael Okpara University of Agriculture, Umudike, Nigeria. E-mail: winnnerkalu@gmail.com; Tel: +234 8060236617
bDepartment of Biochemistry, Federal University, Ndufu-Alike, Ikwo, Ebonyi State, Nigeria
First published on 28th September 2016
Abstract
This study investigated the effect of fractions of kolaviron on some biochemical parameters in benign prostatic hyperplasia (BPH) rats and also characterized the most active fraction (F1) using GC-MS. BPH was induced in the rats by sub-cutaneous injection of dihydrotestosterone and estradiol valerate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) daily for 28 days. Rats were euthanized on the 29th day and analysis was carried out following standard techniques. The body weights, relative prostate weights, serum prostate specific antigen, estradiol and prostatic levels of lipid peroxidation, reduced glutathione, superoxide dismutase, glutathione peroxidase and catalase in the disease control were altered (P < 0.05) compared with the normal control. Finasteride (5 mg/70 kg), kolaviron (200 mg kg−1) or F1 fraction (100 mg kg−1) ameliorated these parameters when compared with the disease the control unlike fractions F2 and F3. Qualitative and quantitative phytochemical assay of the G. kola flour indicated the absence of alkaloids but the presence of flavonoids, cardiac glycosides, tannins and saponins with equivalent composition of 2.67 g/100 g, 3.11 g/100 g, 1.08 g/100 g and 2.35 g/100 g respectively. GC-MS analysis of the F1 fraction indicated the presence of 10 compounds with identified biological activities namely: phenol, 1,2-benzenediol, 2,4,6-trihydroxybenzoic acid, tetradecanoic acid, hexadecanoic acid methyl ester, n-hexadecanoic acid, 9-octadecenoic acid methyl ester, octadecanoic acid, di-n-octyl phthalate and 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl)ester respectively. The anti-BPH properties of the F1 fraction could be attributed to the compounds in it with 5-alpha reductase inhibitory, anti-androgenic and antioxidant properties.
1) daily for 28 days. Rats were euthanized on the 29th day and analysis was carried out following standard techniques. The body weights, relative prostate weights, serum prostate specific antigen, estradiol and prostatic levels of lipid peroxidation, reduced glutathione, superoxide dismutase, glutathione peroxidase and catalase in the disease control were altered (P < 0.05) compared with the normal control. Finasteride (5 mg/70 kg), kolaviron (200 mg kg−1) or F1 fraction (100 mg kg−1) ameliorated these parameters when compared with the disease the control unlike fractions F2 and F3. Qualitative and quantitative phytochemical assay of the G. kola flour indicated the absence of alkaloids but the presence of flavonoids, cardiac glycosides, tannins and saponins with equivalent composition of 2.67 g/100 g, 3.11 g/100 g, 1.08 g/100 g and 2.35 g/100 g respectively. GC-MS analysis of the F1 fraction indicated the presence of 10 compounds with identified biological activities namely: phenol, 1,2-benzenediol, 2,4,6-trihydroxybenzoic acid, tetradecanoic acid, hexadecanoic acid methyl ester, n-hexadecanoic acid, 9-octadecenoic acid methyl ester, octadecanoic acid, di-n-octyl phthalate and 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl)ester respectively. The anti-BPH properties of the F1 fraction could be attributed to the compounds in it with 5-alpha reductase inhibitory, anti-androgenic and antioxidant properties.
1. Introduction
Benign prostatic hyperplasia (BPH) is a common male disease causing lower urinary tract distress in aging men.1 The exact etiology remains a mystery even as its prevalence continues to grow amongst aged men.2 Several factors including inflammatory mediators, hormones, dietary factors, inflammatory genes, and oxidative stress have been implicated in the development of BPH, but there is no consensus as to which is the primary one.3While conventional drugs such as 5α-reductase inhibitors (finasteride and dutasteride) and adrenoceptor antagonists (alfuzosin, doxazosin, tamsulosin, terazosin) are used to treat BPH, the adverse side effects associated with them ranging from impotence and gynaecomastia to orthostatic hypotension and abnormal ejaculation4 have led to increased research for alternative means of managing this disease. Although surgery has also been suggested to be a sure method, the cost and risks associated with it excludes it as a routine treatment.4 The trend then is increased search for alternative methods of managing this disease using herbal medicine.
Following the successes recorded overtime in the management of BPH with phytotherapeutic agents, their popularity grew all over the world especially in China, Japan and Europe.5 In most developing economies, particularly in Africa, herbal medicine constitutes the predominant mode of treating BPH because of the perceived therapeutic healing powers of herbs.6 The major factor that seemed to influence their popularity is the strong belief that they have fewer side effects and less toxic due to their rich natural sources and also, because they are readily available and cheap to obtain.
Phytochemicals are biologically active compounds, found in plants which are not established nutrients but which nevertheless contribute significantly to protection against degenerative diseases.7 These bioactive compounds are known to prevent diseases by inhibiting cellular damage induced by reactive oxygen species.8 In addition, they (phytochemicals) have also been found to be useful in pharmaceutical industries where they are used in drug development which begins with identification of the active principles, detailed biological assays and dosage formulations followed by clinical studies to establish the safety, efficacy and pharmacokinetic profile of the drug.9
Kolaviron, a biflavonoid complex and the major phytochemical extracted from the seeds of Garcinia kola, has been shown to exhibit many pharmacological actions such as anti-atherogenic, anti-diabetic and anti-proliferative properties.10–12
In our previous study,13 we reported the anti-benign prostatic hyperplasia properties of kolaviron. However, the natural products in it that confer it with this biological property have not been reported.
Hence this study which investigated the effect of fractions of kolaviron on the serum levels of prostate specific antigen and estradiol, prostatic antioxidant activities and relative tissue weights of dihydrotestosterone/estradiol valerate-induced benign prostatic hyperplasia in albino rats and also characterized the phytochemical constituents of the fraction with bioactivity using GC-MS technique.
2. Experimental
2.1 Chemicals
Dihydrotestosterone (DHT) and estradiol valerate (ESV) were procured from Sigma-Aldrich Laboratories, Germany and Schering AG, Berlin, Germany, respectively. Finasteride (Gedeon Richter, Hungary) was purchase from Blessed pharmacy, Umuahia, Nigeria. All other reagents were of analytical grade and the purest quality available.2.2 Plant materials
Garcinia kola seeds were obtained locally in Aba, Nigeria. They were authenticated in the Department of Plant Science and Biotechnology, Michael Okpara University of Agriculture, Umudike, Nigeria.2.3 Extract preparation
A total of 3 kg of peeled seeds were sliced, pulverized and then air dried. Extraction of kolaviron from the flours was carried out using the method of Iwu et al.11 Briefly, some quantities of the flours were defatted with light petroleum ether in a Soxhlet apparatus. The defatted dried marc was repacked and then extracted with methanol. The extract was concentrated and diluted to twice its volume with distilled water and extracted with ethylacetate. The concentrated ethylacetate fraction gave a yellow brown solid known as kolaviron.2.4 Qualitative/quantitative phytochemical assay
Some quantities of the flour were subjected to phytochemical screening for the presence of alkaloids, tannins, flavonoids, saponins and cardiac glycosides using the methods of Trease and Evans14 and Harborne15 respectively. The amount of alkaloids in the flour was determined using the method of Harborne.15 Saponins and tannins were determined using the method of AOAC.16 Flavonoids were assayed for using the method of Boham and Kocipai17 while the amount cardiac glycosides in the flour was assayed for using the method of Siddique et al.182.5 Fractionation of kolaviron extract (crude extract)
The kolaviron extract was subjected to column fractionation for purification and partitioning based on the methods of previous researchers.19,20 The sample for the column was prepared by adsorbing 60 g of the kolaviron extract with 100 g of silica gel G (100–200 mesh size). The mixture was air dried and carefully layered on top of the packed silica gel in the column (40 cm length) using a glass funnel. The extract in the column was eluted with ethyl acetate, acetone and methanol respectively in increasing order of polarity.A total of 24 fractions were collected and subjected to thin-layer chromatography (TLC) using silica gel (Kieselgel 60G) coated plates and, solvent mixture of methanol and chloroform in a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v. The RF values were calculated and fractions having the same RF value were bulked together, concentrated to dryness and weighed resulting in three different fractions named as: F1, F2 and F3. Both the kolaviron extract (crude extract) and the fractions were tested in BPH challenged rats for their antiprostatic properties and on the basis of their demonstrated bioactivities, the F1 fraction was further subjected to characterization using the GC-MS technique to determine the active constituents in it that confer it with antiprostatic properties.
4 v/v. The RF values were calculated and fractions having the same RF value were bulked together, concentrated to dryness and weighed resulting in three different fractions named as: F1, F2 and F3. Both the kolaviron extract (crude extract) and the fractions were tested in BPH challenged rats for their antiprostatic properties and on the basis of their demonstrated bioactivities, the F1 fraction was further subjected to characterization using the GC-MS technique to determine the active constituents in it that confer it with antiprostatic properties.
2.6 Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis of the F1 fraction was carried out using the method of Igwe and Okwu21 The components of the extract were identified by matching the peaks with Computer 100 Wiley Ms Libraries and confirmed by comparing the mass spectra of the peaks with those from the database of the National Institute's Standard and Technology (NIST).2.7 Animal protocol
Thirty-five male albino rats (wistar strain), weighing between 90–110 g were used. The animals were acclimatized to their food and water for 2 weeks and fed on standard rat feeds, purchased from Vital feeds, Ibadan, Nigeria. Animals were given access to food and water ad libitum. They were distributed randomly into seven groups of five animals each after approval by the Board of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria which was in line with the guide for the care of use of laboratory animals as given by the National Institute of Health's Principles.222.8 Induction of BPH
BPH was induced in the rats using a mixture of dihydrotestosterone (DHT) and estradiol valerate (ESV) (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The dose for induction of benign prostatic hyperplasia was formulated as 9 mg kg−1 body weight of dihydrotestosterone and 0.9 mg kg−1 body weight estradiol valerate given by subcutaneous injection daily for 28 days.23 Finasteride and kolaviron were administered orally once daily for 28 consecutive days.
1). The dose for induction of benign prostatic hyperplasia was formulated as 9 mg kg−1 body weight of dihydrotestosterone and 0.9 mg kg−1 body weight estradiol valerate given by subcutaneous injection daily for 28 days.23 Finasteride and kolaviron were administered orally once daily for 28 consecutive days.
2.9 Experimental procedure
The rats were divided into 7 groups of 5 rats each to receive the vehicle (olive oil), finasteride, test extracts and hormone for the study period as follows: group 1, olive oil (1 mL kg−1 subcutaneously) + olive oil (1 mL kg−1 orally) (normal control); group 2, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + olive oil (1 mL kg−1 orally) (disease control); group 3, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + finasteride (5 mg/70 kg orally) (reference group), group 4, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + kolaviron (200 mg kg−1 orally) (study group 1); group 5, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + F1 fraction (100 mg kg−1 orally) (study group 2), group 6, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + F2 fraction (100 mg kg−1 orally) (study group 3) and group 7, hormone (9 mg kg−1 DHT + 0.9 mg kg−1 ESV subcutaneously) + F3 fraction (100 mg kg−1 orally) (study group 4). The body weights of the rats were recorded on a daily basis. At the end of 28 days, the rats were subjected to overnight fast and on the 29th day, they were euthanized by cervical dislocation. Blood was collected by cardiac puncture into plain sample tubes and allowed to clot. Sera were harvested from the clotted blood samples following centrifugation at 3000 × g for 20 min for the analysis of prostate specific antigen (PSA). The prostates were excised, washed with cold normal saline, blotted with filter paper, and weighed on an electronic balance (Ohaus, USA).After measurements of the prostatic weights, two prostates were selected from each group and their dorso-lateral lobes were dissected out and processed for histology. The remaining three prostates from each group were homogenized in ice-cold phosphate buffered saline and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 minutes and the supernatants were analyzed for lipid peroxidation, catalase, reduced glutathione (GSH), superoxide disthmutase (SOD) and glutathione peroxidase (GPx) activities.
000 × g for 15 minutes and the supernatants were analyzed for lipid peroxidation, catalase, reduced glutathione (GSH), superoxide disthmutase (SOD) and glutathione peroxidase (GPx) activities.
The histological assay of the prostates was carried out using the method of Ejike and Ezenyika.23 Briefly, the dorsa-lateral lobes of the prostatic tissues were fixed in Bouin's fixative for 24 h. Thereafter, they were dehydrated in grades of ethanol, cleared in xylene, infiltrated with and embedded in paraffin. Each was sectioned at 5 μm and stained with hematoxylin and eosin. The sections were then viewed in a light microscope and photomicrographs were taken.
The percentage change in the body weights of the rats was calculated as:
| {Final body weight − initial body weight}/final body weight} × 100 |
Similarly, the relative prostate weights (g per 1000 g) of the rats were calculated as: {total prostate weight/final body weight} × 1000.
2.10 Assay of prostate specific antigen and estradiol in the sera
The prostate specific antigen in the sera of the rats was determined using enzyme immunoassay (EIA) method as described by Stowell et al.24 Serum levels of estradiol were assayed for using the Biocheck enzyme immunoassay (EIA) kits following the methods described by Bouve et al.252.11 Assay of markers of oxidative stress in the prostate
Catalase activity was determined in the prostates of the rats using the method of Sinha26 SOD activity was determined using the method of Misra and Fridovich.27 Glutathione peroxidase (GPx) was estimated using the method of Paglia and Valentine.28 GSH was determined using the method of Beutler et al.29 Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substance (TBARS) using the method of Varshney and Kale.302.12 Statistical analysis
Data were analyzed statistically using the statistical package for social sciences (SPSS) version 17.0. One-way analysis of variance (ANOVA) was used for comparison of means. Differences between means were considered to be significant when P < 0.05.3. Results and discussion
The body weights, percentage change in body weights and relative prostate weights of the rats investigated in this study is shown in Table 1. The final body weights of the disease control (1.64% increase) were significantly lower (P < 0.05) than that of the normal control that recorded 41.16% increase in body weight. However, the final body weights of the BPH rats administered finasteride (28.51% increase), kolaviron (30.98% increase) or fraction F1 (34.42% increase) were significantly (P < 0.05) increased while that of the BPH rats administered fractions F2 (4.25% increase) or F3 (0.9% increase) were statistically the same (P > 0.05) in comparison with the disease control. The decreased body weights of the disease control could be associated with breakdown of tissue proteins in an attempt to arrest the BPH assault while the increased body weights of the BPH treated rats administered finasteride, kolaviron or fraction F, suggests increased synthesis of tissue proteins in these groups of rats. However, the findings of this study showed that administration of F2 or F3 fractions to the BPH rats of groups 6 and 7 could not reverse their protein loss arising from BPH challenge.| Groups | Initial body weight (g) | Final body weight (g) | Percentage change (increase) | Relative prostate weight (g per 1000 g) |
|---|---|---|---|---|
| a Values are means ± SD. a–d Means with different superscripts along the column are significantly different (P < 0.05). NC – normal control, DC – disease control, H + Fin – hormone + finasteride, H + KV – hormone + kolaviron (200 mg kg−1), H + F1 – hormone + fraction 1 (100 mg kg−1), H + F2 – hormone + fraction 2 (100 mg kg−1), H + F3 – hormone + fraction 3 (100 mg kg−1). | ||||
| NC | 120.92 ± 10.22a | 205.51 ± 12.25c | 41.16 | 0.36 ± 0.05a |
| DC | 119.56 ± 11.16a | 121.55 ± 11.30a | 1.64 | 2.40 ± 0.32d |
| H + Fin | 123.05 ± 8.24a | 172.13 ± 9.57b | 28.51 | 1.13 ± 0.23c |
| H + KV | 122.11 ± 10.94a | 176.91 ± 13.74b | 30.98 | 0.77 ± 0.11b |
| H + F1 | 119.34 ± 12.57a | 181.98 ± 14.50b | 34.42 | 0.61 ± 0.12ab |
| H + F2 | 112.73 ± 16.25a | 117.73 ± 16.13a | 4.25 | 2.16 ± 0.44d |
| H + F3 | 125.82 ± 12.55a | 126.96 ± 10.24a | 0.90 | 2.13 ± 0.40d |
The relative prostate weights of the disease control were significantly increased (P < 0.05) in comparison with the normal control.
Administration of finasteride, kolaviron or fraction F1 to the BPH challenged rats, resulted in significant reduction (P < 0.05) of their prostatic weights in comparison with the disease control, while administration of fractions F2 and F3, did not significantly affect (P > 0.05) the prostatic weights of the BPH challenged rats in comparison with the disease control. Prostate enlargement is used as one of the important markers of BPH.31 Hence, the increased prostatic weight of the disease control is suggestive of prostatic hyperplasia for this group of rats. Finasteride, kolaviron or fraction F1 demonstrated considerable anti-benign prostatic hyperplasia properties which were evident from the reduction of the prostatic growth of the BPH challenged rats following administration of finasteride, kolaviron or fraction F1 at the doses used in this study. In addition, kolaviron or fraction F1 ameliorated the prostatic growth of the BPH rats in a manner akin to finasteride at the dose it was used in this study.
Oxidative stress is considered to be a contributing factor to the development of BPH in elderly men. This is especially true as the human prostate tissue is vulnerable to oxidative DNA damage due to more rapid cell turnover and fewer DNA repair enzymes.3
Lipid peroxidation is the process of oxidative degradation of poly unsaturated fatty acids and the products of lipid peroxidation inactivate cell constituents by oxidation or cause oxidative stress by undergoing radical chain reaction, ultimately leading to cell damage.
The markers of oxidative stress in the prostates of the rats investigated in this study are shown in Table 2. There were significant elevation (P < 0.05) of the prostatic lipid peroxidation but significant reduction (P < 0.05) of the prostatic GSH, SOD, GPx and catalase activities of the disease control compared with the normal control. Administration of finasteride, kolaviron or fraction F1 to the BPH challenged rats, resulted in significant reduction (P < 0.05) of their prostatic lipid peroxidation but significant elevation (P < 0.05) of their prostatic GSH, SOD, GPx and catalase in comparison with the disease control while administration of fractions F2 and F3, did not significantly affect (P > 0.05) the prostatic lipid peroxidation, GSH, SOD, GPx and catalase activities of the BPH challenged rats in comparison with the disease control. The enzymatic antioxidants – SOD, catalase, GPx and glutathione-S-transferase play vital roles during the process of scavenging reactive oxygen species or preventing their formation while the non-enzymatic antioxidant-GSH plays an excellent role in protecting the cell from lipid peroxidation.32 The depletion in the prostatic enzyme antioxidants – SOD, catalase, GPx and non enzymatic GSH of the BPH untreated rats, to the extent that was observed in this study could lead to increased oxidant stress to the prostate with resultant loss of its integrity. This explains the increased lipid peroxidation in their prostates. Finasteride, kolaviron and fraction F1 demonstrated significant antioxidant activities which were evident from the significantly decreased lipid peroxidation but increased SOD, GSH, catalase, GPx and GSH activities of the BPH rats following administration of finasteride, kolaviron or fraction F1 at the doses used in this study. In addition, kolaviron at 200 mg kg−1 or the fraction F1 at 100 mg kg−1 achieved significant improvement in the prostatic antioxidant activity of the BPH induced rats akin to finasteride at 5 mg/70 kg. Our findings on the antioxidant activities of kolaviron lend credence to previous reports of Farombi and Nwaokeafor33 on the antioxidant property of kolaviron. The current study suggests that kolaviron could protect the prostate from oxidative damage arising from BPH.
| Groups | LPO (μ units MDA per mg protein) | GSH (μg g−1 tissue) | Catalase (μmol H2O2 consumed per min per g tissue) | SOD (units per g tissue) | GPx (IU L−1) |
|---|---|---|---|---|---|
| a Values are means ± SD. a–d Means with different superscripts along the column are significantly different (P < 0.05). NC – normal control, DC – disease control, H + Fin – hormone + finasteride, H + KV – hormone + kolaviron (200 mg kg−1), H + F1 – hormone + fraction 1 (100 mg kg−1), H + F2 – hormone + fraction 2 (100 mg kg−1), H + F3 – hormone + fraction 3 (100 mg kg−1). | |||||
| Group 1 (NC) | 0.60 ± 0.17a | 6.87 ± 3.38c | 2.84 ± 0.63d | 0.80 ± 0.10b | 1.56 ± 0.15c |
| Group 2 (DC) | 2.52 ± 0.28c | 1.44 ± 1.00a | 0.50 ± 0.22a | 0.29 ± 0.60a | 0.24 ± 0.07a |
| Group 3 (H + Fin) | 1.59 ± 0.23b | 3.29 ± 1.30b | 1.18 ± 0.44b | 0.75 ± 0.12b | 1.14 ± 0.33b |
| Group 4 (H + KV) | 1.43 ± 0.22b | 4.67 ± 0.84bc | 1.34 ± 0.28bc | 0.69 ± 0.17b | 1.17 ± 0.41b |
| Group 5 (H + F1) | 1.63 ± 0.21b | 5.37 ± 2.04bc | 1.86 ± 0.58c | 0.71 ± 0.12b | 1.23 ± 0.44bc |
| Group 6 (H + F2) | 2.35 ± 0.10c | 1.89 ± 0.56a | 0.48 ± 0.25a | 0.27 ± 0.10a | 0.28 ± 0.10a |
| Group 7 (H + F30) | 2.48 ± 0.27c | 2.33 ± 0.61a | 0.60 ± 0.31a | 0.28 ± 0.07ba | 0.29 ± 0.07a |
The levels of PSA in the sera of the rats investigated in this study are shown in Fig. 1.
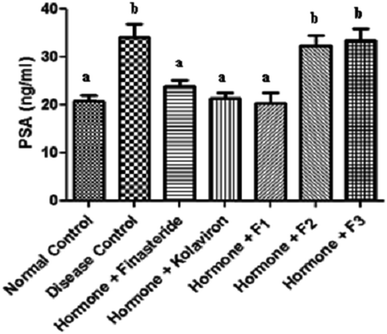 | ||
| Fig. 1 Prostate specific antigen in the sera of rats. Values are reported as means ± SD. a,b Means with different superscripts are significantly different for each group (p < 0.05). | ||
There was a significant elevation of the serum levels of PSA in the disease control (P < 0.05) in comparison with the normal rats.
Administration of finasteride, kolaviron or fraction F1 to the BPH challenged rats, resulted in significant (P < 0.05) decrease of their serum levels of PSA in comparison with the disease control, while administration of fractions F2 and F3, did not significantly affect (P > 0.05) their serum PSA levels when compared with the disease control. PSA is a glycoprotein produced in low quantities by cells of the prostate gland and present in the serum which could be used as semi-quantitative indicator or marker for BPH and prostatic cancer.34 A decrease in PSA is linked to a reduction in prostatic hyperplasia due to inhibition of prostatic 5α-reductase, the enzyme that converts testosterone to dihydrotestosterone (DHT).35 Therefore, the decreased serum PSA levels of the BPH rats following administration of finasteride, kolaviron or fraction F1 further indicates the anti-benign prostatic hyperplasia properties of kolaviron or fraction F1 at the dose they were used in this study. Furthermore, kolaviron or fraction F1 also improved the serum PSA levels of the BPH challenged rats in a manner akin to finasteride at the dose it was used in this study.
The serum levels of estradiol in the sera of the rats investigated in this study are shown in Fig. 2. The estradiol levels of the disease control were significantly elevated (P < 0.05) when compared with the normal control. While administration of finasteride, kolaviron or fraction F1 significantly modulated (P < 0.05) the estradiol levels of the BPH rats, administration of fractions F2 and F3 did not significantly affect (P > 0.05) the estradiol levels of the BPH rats in comparison with the disease control.
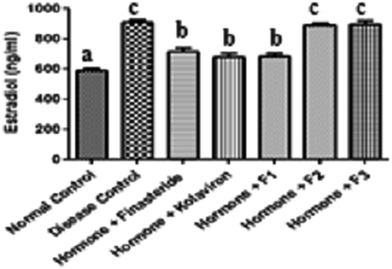 | ||
| Fig. 2 Estradiol levels in the sera of rats. Values are reported as means ± SD. a–c Means with different superscripts are significantly different for each group (p < 0.05). | ||
During ageing, testicular function in elderly men has been reported to be decreased, as are androgen levels. Furthermore, the conversion of androgen to estrogen is increased.36 The released estrogens cause increased stimulation of the prostatic stroma, resulting in excessive proliferation of the prostate and occurrence of BPH.
The increased levels of estradiol in the sera of the BPH untreated rats suggests that the exogenous dihydrotestosterone/estradiol valerate that was administered to these group of rats might have resulted in their increased synthesis of estradiol. On the other hand, the decreased serum levels of estradiol in the BPH rats administered finasteride, kolaviron or fraction F1 further support their usefulness in the management of BPH. This increase in the serum levels of estradiol in the BPH rats could not be ameliorated by administration of fractions F2 or F3 to the BPH rats.
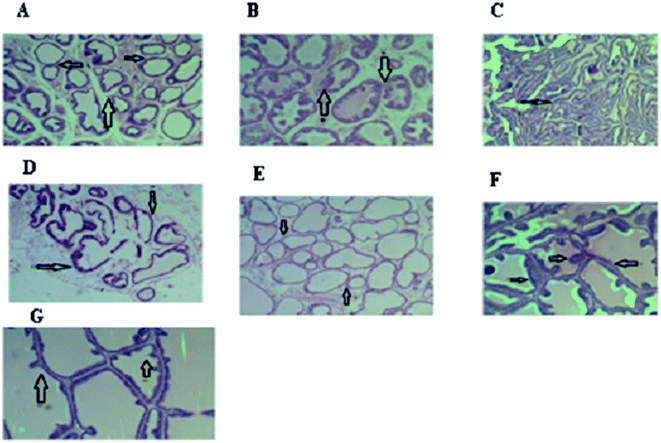
The histology of the prostates of the rats investigated in this study is shown in Fig. 3.
The histology of the prostatic tissue of the normal rats as shown in (a) revealed normal acini cells with scanty secretion and no structural changes. Acini of variable diameter were observed to be normal in this group. Overall histology for this group suggested normal prostate gland.
Histology of the prostatic tissue of the disease control (b) indicated hyperplasia and hypertrophy of the acini cells with the acini filled up with increased secretions. The walls of tubules were thickened and almost every tubule developed large involutions projecting into the lumen, reducing the volume of the lumen compared with the control group. The shape of the tubules was obliterated. Overall histology for this group revealed prostatic hyperplasia.
In the BPH rats treated with finasteride (c), hyperplastic acini cells were observed to have become necrotic with collapsed acini, leading to reduction in size while some still contained the secretion.
In the BPH rats treated with kolaviron (d), there was necrosis of the walls of the acini. The acini were devoid of secretion. In some cases, the wall of the acini was found to be broken down.
Histology of the prostatic tissue of the BPH rats treated with fraction F1 (e) showed necrotic cells but lack of secretion into the acini. The acini were also not collapsed.
For the BPH rats treated with fraction F2 (f), histology of their prostatic tissue showed hyperplasia and hypertrophied acini cells, very active secretions with filled up acini.
For the BPH rats treated with fraction F3 (g), there were also evidences of hyperplasia and hypertrophy of the acini cells with hyper secretion into the cells. The histological findings showed the recovery of the prostatic histoarchitecture particularly the cuboidal epithelial cells, intracellular lumen and shape which support kolaviron and the fraction F1 as strong candidates for the management of prostatic hyperplasia unlike fractions F2 and F3 that did not produce any therapeutic effects.
The result of the qualitative screening of the phytochemicals present in the G. kola flour is shown in Table 3. Results showed the absence of flavonoids but the presence of flavonoids, tannins, saponins and cardiac glycosides in the flour.
| Components | Presence |
|---|---|
| a +: present, ++: highly present, −: absent. | |
| Alkaloids | − |
| Flavonoids | ++ |
| Tannins | + |
| Saponins | ++ |
| Cardiac glycosides | ++ |
The quantitative assay of the phytochemicals present in the G. kola flour as shown in Table 4 revealed that the flour contained 2.67 g/100 g flavonoids, 3.11 g/100 g glycosides, 1.08 g/100 g tannins and saponins (2.35 g/100 g). These findings which showed that Garcinia kola flour contained considerable amounts of these phytochemicals, agree with previous reports of Adegboye et al.37 Furthermore, the considerable amount of the polyphenols (flavonoids and tannins) in the flour could have contributed to the antioxidant activity of this plant as demonstrated in this study. This is owing to the fact that these polyphenol compounds have been reported to confer medicinal plants with antioxidant properties due to their redox and metal chelating properties.38
| Parameters | Composition (g per 100 g) |
|---|---|
| a Values are expressed as means ± SD. | |
| Flavonoids | 2.67 ± 0.54 |
| Saponins | 2.35 ± 0.16 |
| Tannins | 1.08 ± 0.10 |
| Cardiac glycosides | 3.11 ± 0.20 |
GC-MS analysis has proven to be a reliable technique for the identification of the bioactive constituents of medicinal plants.39
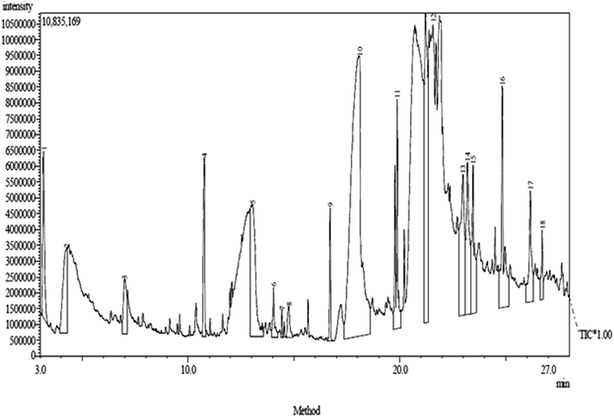
The GC-MS chromatogram of the F1 fraction revealed the presence of eighteen compounds with corresponding peaks at different retention times (Fig. 4). The compounds identified in the extract as shown in Table 5 were as follows:
| Peak no. | Compound name | Molecular formula (MF) | Molecular weight | Retention time (RT) | (%) content |
|---|---|---|---|---|---|
| 1 | 2-Butoxyethanol | C6H14O2 | 118 | 3.167 | 3.20 |
| 2 | Phenol | C6H6O | 94 | 4.267 | 4.17 |
| 3 | 1,2-Benzenediol | C6H6O2 | 110 | 6.998 | 2.05 |
| 4 | 2-Hydroxy-3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one | C10H16O2 | 168 | 10.770 | 2.95 |
| 5 | 2,4,6-Trihydroxybenzoic acid | C7H6O5 | 170 | 13.028 | 7.51 |
| 6 | Tetradecanoic acid | C14H28O2 | 228 | 14.032 | 0.98 |
| 7 | 1-Tetradecene | C14H28 | 196 | 14.427 | 0.23 |
| 8 | 3-Acetyl-5,7-octadien-2-one | C10H14O2 | 166 | 14.756 | 0.86 |
| 9 | Hexadecanoic acid methyl ester | C17H34O2 | 270 | 16.710 | 1.56 |
| 10 | n-Hexadecanoic acid | C16H32O2 | 256 | 18.080 | 33.09 |
| 11 | 9-Octadecenoic acid methyl ester | C19H36O2 | 296 | 19.791 | 5.04 |
| 12 | Octadecanoic acid | C18H36O2 | 284 | 21.221 | 11.54 |
| 13 | 9-Octadecynoic acid | C18H32O2 | 280 | 22.985 | 6.60 |
| 14 | Eicosanoic acid | C20H40O2 | 312 | 23.200 | 5.64 |
| 15 | Hexanedioic acid dioctyl ester | C22H42O2 | 370 | 23.461 | 4.10 |
| 16 | Di-n-octyl phthalate | C24H38O4 | 390 | 24.847 | 5.98 |
| 17 | (E)-13-Docosenoic acid | C22H42O2 | 338 | 26.169 | 3.30 |
| 18 | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl)ester | C24H38O4 | 390 | 26.742 | 1.19 |
The first compound was identified to be 2-butoxyethanol and it constituted 3.20% of the F1 fraction.
The second compound was identified to be phenol and it constituted 4.17% of the fraction.
Compound 3 was identified to be 1,2-benzenediol and it constituted 2.05% of the fraction.
Compound 4 was identified to be 2-hydroxy-3-methyl-6-(1-methylethyl)-2-cyclohexen-1-one and it constituted 2.95% of the fraction.
Compound 5 was identified as 2,4,6-trihydroxybenzoic acid and it constituted 7.51% of the fraction.
Compound 6 was identified as tetradecanoic acid and it constituted 0.98% of the fraction.
Compound 7 was identified as 1-tetradecene and it constituted 0.23% of the fraction.
Compound 8 was identified as 3-acetyl-5,7-octadien-2-one and it constituted 0.86% of the fraction.
Compound 9 was identified as hexadecanoic acid methyl ester and it constituted 1.56% of the fraction.
Compound 10 was identified as n-hexadecanoic acid and it constituted 33.09% of the fraction.
Compound 11 was identified as 9-octadecenoic acid methyl ester and it constituted 5.04% of the fraction.
Compound 12 was identified as octadecanoic acid and it constituted 11.54% of the fraction.
Compound 13 was identified as 9-octadecynoic and it constituted 6.60% of the fraction.
Compound 14 was identified as eicosanoic acid and it constituted 5.64% of the fraction.
Compound 15 was identified as hexanedioic acid dioctyl ester and it constituted 4.1% of the fraction.
Compound 16 was identified as di-n-octyl phthalate and it constituted 5.98% of the fraction.
Compound 17 was identified as (E)-13-docosenoic acid and it constituted 3.3% of the fraction.
Compound 18 was identified as 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl)ester and it constituted 1.19% of the fraction.
The major components of the F1 fraction with identified biological activities were: phenol, 1,2-benzenediol, 2,4,6-trihydroxybenzoic acid, tetradecanoic acid, hexadecanoic acid methyl ester, n-hexadecanoic acid, 9-octadecenoic acid methyl ester, octadecanoic acid, di-n-octyl phthalate and 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl)ester respectively, making up 73.11% of the F1 fraction. The remaining identified compounds that made up 26.89% of the fraction had unknown biological activities.
According to Huang et al.40 phenol which constituted 4.17% of fraction F1 possesses anti-oxidant, anti-carcinogenic, anti-mutagenic and anti-inflammatory properties. Therefore, the presence of this compound in the F1 fraction suggests it could have some antioxidant, anti-carcinogenic, anti-mutagenic and anti-inflammatory properties.
1,2-Benzenediol that constituted 2.05% of fraction F1 was reported to possess antioxidant and anti-carcinogenic properties.41 Therefore, the presence of this compound in fraction F1 further suggests its antioxidant and anti-carcinogenic properties.
According to Feng-lin et al.42 2,4,6-trihydroxybenzoic acid which constituted 7.51% of fraction F1 possesses anti-inflammatory properties. Thus, the presence of this compound in the F1 fraction could confer it with some anti-inflammatory properties.
Tetradecanoic acid that constituted 0.98% of fraction F1 was reported to possess hypocholesterolemic, anti-oxidant, and anti-carcinogenic properties.43 The presence of this compound in this fraction could confer it with hypocholesterolemic actions while it further suggests the anti-oxidant and anticarcinogenic properties of this F1 fraction.
Hexadecanoic acid methyl ester that constituted 1.56% of fraction F1 was reported to possess anti-oxidant, anti-androgenic, hypocholesterolemic and 5-alpha reductase inhibitory properties.44,45 Therefore, the presence of this compound in this fraction suggests it could possess biological properties.
n-Hexadecanoic acid that constituted 33.09% of fraction F1 was reported to possess anti-oxidant, anti-androgenic, hypocholesterolemic and 5-alpha reductase inhibitory properties.44,46 The presence of this compound as the major natural product identified in this fraction suggests the anti-androgenic and hypocholesterolemic property of this F1 fraction and also provides a biochemical rationale for the anti-oxidant and PSA inhibitory (decrease in PSA is associated with inhibition of prostatic 5α-reductase) property of this fraction as observed in this study.
9-Octadecenoic acid methyl ester that constituted 5.04% of the F1 fraction was reported to possess anticarcinogenic properties.47,48 The presence of this compound in the F1 fraction suggests the F1 fraction could possess anti carcinogenic properties.
According to Basu et al.43 octadecanoic acid that constituted 11.54% of fraction F1 possesses 5-alpha reductase inhibitory and hypocholesterolemic properties. The presence of this compound also suggests the hypocholesterolemic action of fraction F1 and further affirms its 5-alpha reductase inhibitory property.
Di-n-octyl phthalate that constituted 5.98% of the F1 fraction was reported to possess anti snake venom properties.49 The presence of this compound in this fraction suggests it could possess anti-snake venom properties.
According to Saranya et al.50 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl)ester that constituted 1.19% of the F1 fraction possesses antimicrobial and anti-inflammatory actions. The presence of this compound in this fraction could confer it with these biological properties.
Going by the findings of this study, it is plausible to attribute the anti-benign prostatic hyperplasia properties of the F1 fraction of kolaviron to the bioactive compounds in it with 5-alpha reductase inhibitory, anti-androgenic and antioxidant properties. These compounds could work in synergy to confer this fraction with its anti-benign prostatic hyperplasia properties although further studies will be needed to confirm this.
4. Conclusions
The current study revealed that the anti-benign prostatic hyperplasia properties of the F1 fraction of kolaviron could be linked to the bioactive compounds in it with 5-alpha reductase inhibitory, anti-androgenic and antioxidant properties.Notes and references
- Y. J. Lee, S. J. Jeong, S. S. Byun, J. J. Lee, J. W. Han and K. W. Kim, Korean J. Urol., 2012, 53, 263–267 CrossRef PubMed
.
- S. P. Stroup, K. Palazzi-Churas, R. P. Kopp and J. K. Parsons, BJU Int., 2012, 109, 84–87 CrossRef PubMed
.
- P. L. Minciullo, A. Inferrera, M. Navarra, G. Calapai, C. Magno and S. Gangemi, Urol. Int., 2015, 94, 249–254 CrossRef CAS PubMed
.
- M. Oelke, M. A. Kuczyk and T. R. Herrmann, Der Urologe, 2009, 48, 1365–1377 CrossRef CAS PubMed
.
- L. Zegarra, A. Vaisberg, C. Loza, M. Aguirre, R. L. Campos, I. Fernandez, O. Talla and L. Villegas, Int. Braz. J. Urol., 2007, 33, 493–501 CrossRef PubMed
.
- T. Odugbemi, Outlines and Pictures of Medicinal Plants in Nigeria, University of Lagos Press, Nigeria, 2006, pp. 55–71 Search PubMed
.
- I. E. Dreosti, Asia Pac. J. Clin. Nutr., 2000, 9, 119–122 CrossRef
.
- S. H. Hung, C. W. Yu and C. H. Lin, Bot. Bull. Acad. Sin., 2005, 64, 1–10 Search PubMed
.
- C. O. Eleazu, Pharm. Biol., 2016, 1–6 CrossRef CAS PubMed
.
- O. A. Adaramoye, V. O. Nwaneri, K. C. Anyanwu, E. O. Farombi and G. O. Emerole, Clin. Exp. Pharmacol. Physiol., 2005, 32, 40–46 CrossRef CAS PubMed
.
- M. M. Iwu, O. A. Igbok, C. O. Okunji and M. S. Tempesta, J. Pharm. Pharmacol., 1990, 42, 290–292 CrossRef CAS PubMed
.
- E. O. Farombi and O. Olatunde, Int. J. Environ. Res. Public Health, 2011, 8, 2533–2555 CrossRef PubMed
.
- W. O. Kalu, P. N. Okafor, I. I. Ijeh and C. Eleazu, Biomed. Pharmacother., 2016, 83, 1436–1443 CrossRef CAS PubMed
.
- G. E. Trease and W. C. Evans, Pharmacognosy, ed. I. Yukari and F. Youichi, Brown Publications, Ikuko, 14th edn, 1983 Search PubMed
.
- J. B. Harborne, Phytochemical Methods, Chapman and Hall, Ltd, London, 1973, p. 113 Search PubMed
.
- AOAC, Official Methods of Analysis, Association of Official Analytical Chemists, Arlington, VA, 15th edn, 1990 Search PubMed
.
- A. B. Boham and A. C. Kocipai, Pac. Sci., 1974, 48, 458–463 Search PubMed
.
- S. Siddique, F. Hafeez and B. Beggum, Phytochemistry, 1987, 26, 237–241 CrossRef
.
- T. A. Beek and G. P. Lelyveld, J. Nat. Prod., 1997, 60, 735–738 CrossRef
.
- A. E. Effiong, P. Ebong and A. O. Eseyin, Int. J. Biochem. Biotechnol., 2013, 2, 457–460 Search PubMed
.
- O. U. Igwe and D. E. Okwu, Asian J. Plant Sci. Res., 2013, 3, 47–54 CAS
.
- National Research Council (NRC), Guide for the Care and Use of Laboratory Animals, National Institute of Health, Bethesda (MD), 1985, p. 8523 Search PubMed
.
- C. E. C. C. Ejike and L. U. S. Ezeanyika, Int. J. Curr. Res., 2010, 6, 065–067 Search PubMed
.
- L. I. Stowell, I. E. Sharman and K. Hamel, Forensic Sci. Int., 1991, 50, 125–138 CrossRef CAS PubMed
.
- J. Bouve, J. De Boever, D. Leyseele, E. Bosmans and P. Dubois, Direct enzyme immunoassay of estradiol in serum of women enrolled in an in vitro fertilization and embryo transfer program, Clin. Chem., 1992, 38, 1409–1413 CAS
.
- A. K. Sinha, Anal. Biochem., 1972, 47, 389–394 CrossRef CAS PubMed
.
- H. P. Misra and I. Fridovich, J. Biol. Chem., 1972, 247, 188–192 CAS
.
- D. E. Paglia and W. N. Valentine, J. Lab. Clin. Med., 1967, 70, 158 CAS
.
- E. Beutler, O. Duron and B. M. Kelly, J. Lab. Clin. Med., 1963, 61, 882–888 CAS
.
- R. Varshney and R. K. Kale, Int. J. Radiat. Biol., 1990, 58, 733–743 CrossRef CAS PubMed
.
- P. Chiung-Chi, L. Jia-Hong, C. Chi-Huang, C. Jin-Yuan, C. Kuan-Chou, C. Kuang-Yu and Y. P. Robert, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 1–12 Search PubMed
.
- P. Leelavinothan, K. Asaithambi and R. Ayyasamy, Toxicol. Rep., 2015, 2, 46–55 CrossRef
.
- E. O. Farombi and I. A. Nwaokeafor, Clin. Exp. Pharmacol. Physiol., 2005, 32, 667–674 CrossRef CAS PubMed
.
- J. Zhou, L. Jiumao, W. Xu, Z. Xiaoyong and Z. Yuqing, Qianliening capsule treats benign prostatic hyperplasia through regulating the expression of sex hormones, estrogen receptor and androgen receptor, Afr. J. Pharm. Pharmacol., 2010, 6, 173–180 CrossRef
.
- J. M. McPartland and P. L. Pruitt, J. Am. Osteopath. Assoc., 2000, 100, 89–96 CAS
.
- J. D. McConnell, J. D. Wilson, F. W. George, J. Geller, F. Pappas and E. Stoner, J. Clin. Endocrinol. Metab., 1992, 74, 505–508 CAS
.
- M. F. Adegboye, D. A. Akinpelu and A. I. Okoh, Afr. J. Biotechnol., 2008, 7, 3934–3938 CAS
.
- C. O. Eleazu and K. C. Eleazu, Am. J. Food Technol., 2012, 7, 214–221 CrossRef CAS
.
- Z. Cong, Q. Meiling, S. Qinglong, Z. Shan and F. Ruonong, J. Pharm. Biomed. Anal., 2007, 44, 464–470 CrossRef PubMed
.
- W. Y. Huang, Y. Z. Cai and Y. Zhang, Nutr. Cancer, 2010, 62, 1–20 CrossRef CAS PubMed
.
- M. S. Manorenjitha, A. K. Norita, S. Norhisham and M. Z. Asmawi, Int. J. Pharma Biosci. Technol., 2013, 4, 99–103 CAS
.
- H. Feng-lin, Y. Li-ming, C. Shwu-fen, W. Li-hsuan, H. Chung-yi, L. Pan-chun and L. Shwu-jiuan, Appl. Microbiol. Biotechnol., 2007, 74, 659–666 CrossRef PubMed
.
- S. Basu, R. C. Utpal, D. Mounita and D. Gouriprosad, Int. J. Phytomed., 2013, 5, 243–251 CAS
.
- M. Sermakkani and V. Thangapandian, Asian J. Pharm. Clin. Res., 2012, 5, 90–94 CAS
.
- T. Sudha, S. Chidambarampillai and V. R. Mohan, J. Appl. Pharm. Sci., 2013, 3, 126–130 Search PubMed
.
- R. K. Jananie, V. Priya and K. Vijayalakshmia, New York Science Journal, 2011, 4, 16–20 Search PubMed
.
- F. A. Syeda, R. Habibur, M. I. Choudahry and R. Attaur, Int. J. Genet. Mol. Biol., 2011, 3, 95–100 Search PubMed
.
- L. H. Yeong, K. G. Nancy and W. P. Micheal, J. Agric. Food Chem., 1989, 37, 75–81 CrossRef
.
- S. Ibrahim, J. A. Nok, M. S. Abubakar and S. Sarkiyayi, Res. J. Appl. Sci., Eng. Technol., 2012, 4, 2382–2387 CAS
.
- D. K. Saranya, P. B. Sruthy, J. C. Anjana, J. Rathinamala and S. Jayashree, Int. J. Appl. Biol. Pharm. Technol., 2013, 4, 272–276 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |