Open Access Article
Open Access ArticleNew one-pot method for the synthesis of pyrrolidinofullerenes†
A. R.
Tuktarov
*a,
Z. R.
Shakirova
a,
Yu. G.
Budnikova
b,
R. B.
Salikhov
c and
U. M.
Dzhemilev
a
aInstitute of Petrochemistry and Catalysis, Russian Academy of Sciences, Russian Federation. E-mail: tuktarovar@gmail.com
bArbuzov Institute of Organic and Physical Chemistry, Kazan Research Center, Russian Academy of Sciences, Russian Federation
cBashkir State University, Russian Federation
First published on 23rd August 2016
Abstract
The reaction of fullerene C60 with isocyanoacetates and EtMgBr in the presence of stoichiometric amounts of Ti(Oi-Pr)4 was studied for the first time. Unlike esters and nitriles of carboxylic acids and isonitriles, isocyanoacetates were found to react with C60 under the developed conditions to give N-unsubstituted pyrrolidinofullerenes. The electrochemical reduction of the C60 derivatives we synthesized was found to proceed less easily than that of C60 but more easily than that of unsubstituted pyrrolidinofullerenes.
Introduction
The interest in pyrrolidinofullerenes is caused by their potential applications in medicine,1 in electronics and non-linear optics2 and as organic ferromagnets,3 photosensitizers in the generation of singlet oxygen,4 molecular switches,5 and photoconverters of solar energy.6The key method for the synthesis of pyrrolidinofullerenes is 1,3-dipolar cycloaddition of azomethine ylides generated in situ to fullerenes (Prato reaction), which gives the target fulleroheterocycles in preparative yields.7 Alternative methods include photochemical8 and catalytic9 cycloaddition of tertiary amines to C60, but these methods have not received wide use in fullerene chemistry because of low yields of the target adducts or the necessity to use starting amines of a particular structure.
Recently,10 we developed a novel original method for functionalization of fullerene C60 with various functional compounds and EtMgBr in the presence of Ti(Oi-Pr)4, which gave, depending on the reactant structure, various acyclic or cyclic C60 derivatives difficult to prepare by other methods. For example, esters of aromatic carboxylic acids give fullerenyl ketones in this reaction,10a nitriles afford previously unknown fullerotetrahydropyridines,10b and cyanoacrylates and isonitriles are converted to aminomethanofullerenes previously difficult to obtain.10c,d
As a continuation of this study, we investigated the reaction of C60 with isocyanoacetates under the previously developed conditions.10
Result and discussion
Resorting to published data,10–12 we assumed that isocyanoacetates containing divalent carbon in the carbene canonical form and an active methylene group between the isonitrile and ester groups would react with fullerene C60 and EtMgBr in the presence of the Ti(Oi-Pr)4 catalyst to give pyrrolidinofullerenes, which are difficult to obtain by other methods, according to the following chart:We found that the reaction of model methyl isocyanoacetate with fullerene C60 and EtMgBr proceeds under argon in the presence of Ti(Oi-Pr)4 in chlorobenzene at 80 °C at the C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isocyanoacetate
isocyanoacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Mg]
[Mg]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ti] ratio of 1
[Ti] ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and gives, after hydrolysis of the reaction mixture with 5% aqueous HCl, a mixture of pyrrolidinofullerene stereoisomers 1a and 1b in a ∼65% total yield (in relation to taken C60) and in 1
3 and gives, after hydrolysis of the reaction mixture with 5% aqueous HCl, a mixture of pyrrolidinofullerene stereoisomers 1a and 1b in a ∼65% total yield (in relation to taken C60) and in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, respectively (Scheme 1). A change in the ratio of the reactant and catalyst components and a decrease in the reaction temperature result in a sharp decrease of the total yield of target pyrrolidinofullerenes 1a,b (8–15%) and in the formation of side products via C60 carbomagnesiation and hydrogenation.
2 ratio, respectively (Scheme 1). A change in the ratio of the reactant and catalyst components and a decrease in the reaction temperature result in a sharp decrease of the total yield of target pyrrolidinofullerenes 1a,b (8–15%) and in the formation of side products via C60 carbomagnesiation and hydrogenation.
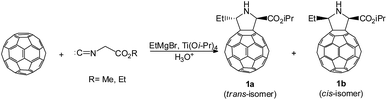 | ||
| Scheme 1 The reaction of methyl or ethyl isocyanoacetates with fullerene C60 and EtMgBr in the presence of Ti(Oi-Pr)4. | ||
The results obtained using ethyl isocyanoacetate rather than methyl isocyanoacetate were similar.
Pyrrolidinofullerenes 1a,b were isolated from the reaction mixture by means of preparative HPLC. According to 1D (1H and 13C) and 2D (HHCOSY, HSQC, HMBC) NMR experiments and IR and MALDI TOF MS data, the reaction under conditions we developed affords a mixture of stereoisomeric cycloadducts a and b.
Indeed, the 13C NMR spectrum of a mixture of stereoisomers 1a,b shows a doubled set of signals with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 intensity ratio both for carbon atoms of the fullerene cage and for the attached addend. The intense signals at δC 79.38 and 78.34 ppm correspond to the sp3 hybridized atoms of the fullerene cage of cis-isomer 1b, while the methine carbon atoms of the pyrrolidine ring are responsible for higher-field signals at δC 74.20 and 75.24 ppm, correlated with the nitrogen signal in the HMBC (NH) experiment, δN 60.96 ppm. For trans-isomer 1a, the spectrum exhibits a set of similar signals of low intensity, belonging to the sp3 hybridized carbon atoms of C60 (δC 76.77 and 77.58 ppm), methine carbon atoms of the heterocycle (δC 72.32 and 74.03 ppm), and the nitrogen atom (δN 55.17 ppm). The C(3′) methine carbon signals of cis-(δC 75.24 ppm) and trans-(δC 74.03 ppm) isomers also show cross-peaks with the signals of the ethyl-group methylene protons (δH 2.20 and 2.32 ppm, respectively) in the HMBC spectra. The cis-configuration of the 1′,3′ substituents in 1b was unambiguously proved by the cross-peaks in the NOESY experiment between the methine proton signals at δH (1′) 5.43 ppm and δH (3′) 4.68 ppm, whereas trans-isomer 1a does not show this cross-peak.
2 intensity ratio both for carbon atoms of the fullerene cage and for the attached addend. The intense signals at δC 79.38 and 78.34 ppm correspond to the sp3 hybridized atoms of the fullerene cage of cis-isomer 1b, while the methine carbon atoms of the pyrrolidine ring are responsible for higher-field signals at δC 74.20 and 75.24 ppm, correlated with the nitrogen signal in the HMBC (NH) experiment, δN 60.96 ppm. For trans-isomer 1a, the spectrum exhibits a set of similar signals of low intensity, belonging to the sp3 hybridized carbon atoms of C60 (δC 76.77 and 77.58 ppm), methine carbon atoms of the heterocycle (δC 72.32 and 74.03 ppm), and the nitrogen atom (δN 55.17 ppm). The C(3′) methine carbon signals of cis-(δC 75.24 ppm) and trans-(δC 74.03 ppm) isomers also show cross-peaks with the signals of the ethyl-group methylene protons (δH 2.20 and 2.32 ppm, respectively) in the HMBC spectra. The cis-configuration of the 1′,3′ substituents in 1b was unambiguously proved by the cross-peaks in the NOESY experiment between the methine proton signals at δH (1′) 5.43 ppm and δH (3′) 4.68 ppm, whereas trans-isomer 1a does not show this cross-peak.
The formation of stereoisomeric pyrrolidine cycloadducts 1a,b of the composition C68H15NO2 was confirmed by the MALDI TOF mass spectrometry. The experiments were performed in the linear (TOF) and reflection (TOF/TOF) modes and in both positive and negative ion modes using elemental sulfur as the matrix and showed the molecular ion [M + H] peak, m/z 878.044 (Mcalcd = 877.110).
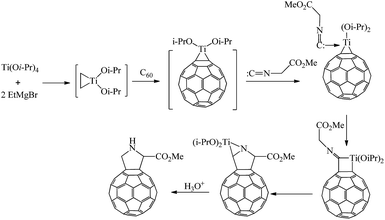
According to the published11,12 and our10 experimental data, we assumed a probable pathway for the formation of pyrrolidinofullerene 1 in the reaction in question (Scheme 2). In the first step, Ti(Oi-Pr)4 reacts with EtMgBr to give dialkoxytitanocyclopropane occurring in equilibrium with the ethylene complex. Fullerene present in the reaction mixture displaces the ethylene molecule from the complex to afford the key reaction intermediate, namely, fullerotitanacyclopropane A. The subsequent reaction of intermediate A with the isocyanoacetate at the metal–carbon bond yields titanacyclobutane B. Demetallation of B under the reaction conditions and subsequent transformations involving the reactive methylene group of the starting isonitrile result in the formation of pyrrolinofullerene C. In view of the excess of EtMgBr and Ti(Oi-Pr)4 in the reaction mixture, the intermediate C undergoes carbomagnesiation of the heterocyclic moiety at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with simultaneous transesterification of the ester group, being thus converted to complex D, which is hydrolyzed to furnish the target pyrrolidinofullerene 1.
N bond with simultaneous transesterification of the ester group, being thus converted to complex D, which is hydrolyzed to furnish the target pyrrolidinofullerene 1.
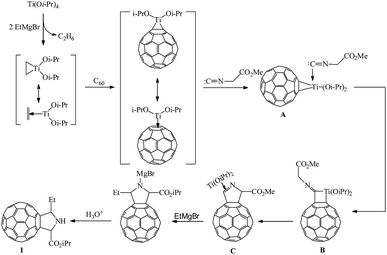 | ||
| Scheme 2 Probable pathway for the formation of pyrrolidinofullerenes in the reaction of isocyanoacetates with fullerene C60 and EtMgBr in the presence of Ti(Oi-Pr)4. | ||
In view of the fact that the structure of substituents in the pyrrolidine ring of the C60 derivative depends on the structure of the Mg organic compound, we carried out this reaction under the developed conditions using a variety of alkyl- and arylmagnesium bromides (Scheme 3, Table 1). More bulky structure of substituent in the initial organomagnesium compound was found to lead to higher proportions of trans-fulleropyrrolidines, up to the selective formation of the trans-isomers in the case of PhMgBr. The introduction of a substituent into the aromatic ring of the organomagnesium compound reduced the selectivity, and the reaction gave a mixture of stereoisomeric pyrrolidinofullerenes 6 with a cis to trans ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating that the electronic effects as well as the steric effects influence on selectivity of formation of the cis,trans isomers.
2, indicating that the electronic effects as well as the steric effects influence on selectivity of formation of the cis,trans isomers.
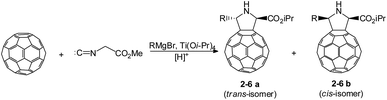 | ||
| Scheme 3 Variation of alkyl- and arylmagnesium bromides in the reaction of C60 with methyl isocyanoacetate. | ||
| Entry | R | Product | Yield, % | Ratio | |
|---|---|---|---|---|---|
| a | b | ||||
| 1 | Bu | 2 | 50 | 2 | 3 |
| 2 | i-Pr | 3 | 55 | 1 | 1 |
| 3 | Cy | 4 | 51 | 8 | 10 |
| 4 | Ph | 5 | 45 | 1 | 0 |
| 5 | o-MePh | 6 | 43 | 2 | 1 |
As model compounds, we chose phenyl-substituted fulleropyrrolidine 5 and study the electrochemical reduction.
Fulleropyrrolidine 5 can reversibly add four electrons in four steps (Fig. 1) to give stable multianions:
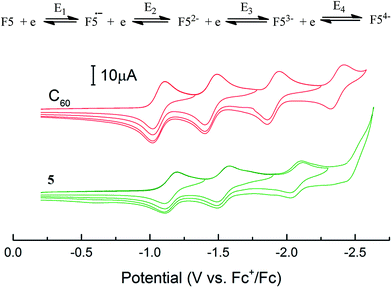 | ||
| Fig. 1 Cyclic voltammograms of C60 and its novel derivative 5 (10−3 M in 1,2-dichlorobenene, Bu4NBF4). | ||
The first reduction potentials of functionally substituted fullerene 5 are somewhat more negative than that of fullerene C60, which is caused by the effect of substituents (Table 2). The fourth reduction peak is is nearly merged with the background. The stability of the electrochemically reduced fullerene species (radical anion formed in the first step and polyanions formed in the subsequent steps) can be evaluated from the ratio of anodic and cathodic currents in the ipa/ipc peak (Table 2).
| Comp. | Peak # | E pc | E pa | i pa/ipc | ΔE | E 1/2 | E LUMO |
|---|---|---|---|---|---|---|---|
| a The data for C60 are close to reported values.13 obtained under similar conditions. Due to proximity of the fourth peak to the supporting electrolyte discharge line, the calculation of ipa/ipc is in some cases not appropriate (this is designated as N/A). The LUMO energy (eV) was calculated as ELUMO = −(E[1/2,red vs. Fc+/Fc] + 4.8) (eV).14 | |||||||
| 5 | 1 | −1.20 | −1.11 | 0.73 | 0.09 | −1.16 | −3.65 |
| 2 | −1.58 | −1.49 | 1.00 | 0.09 | −1.54 | −3.27 | |
| 3 | −2.12 | −2.02 | 1.00 | 0.10 | −2.07 | −2.73 | |
| 4 | −2.54 | N/A | N/A | N/A | N/A | N/A | |
| C60a | 1 | −1.11 | −1.02 | 0.95 | 0.09 | −1.07 | −3.74 |
| 2 | −1.49 | −1.40 | 1.00 | 0.09 | −1.45 | −3.36 | |
| 3 | −1.95 | −1.86 | 1.00 | 0.09 | −1.91 | −2.9 | |
| 4 | −2.42 | −2.32 | 0.76 | 0.1 | −1.37 | −2.43 | |
It is known that the effect of substituents present in the C60 molecule on the reduction potentials is composed of two effects, namely, the change in the electronic properties of the proper fullerene sphere as a result of disruption of the common π-system of conjugation and the electron-donating or -withdrawing properties of the substituents. For example, the substituents at the exo-carbon atom of C60 in methanofullerene 2 are not involved in the common fullerene π-system, which can be regarded as the reaction center of the electron transfer by two σ-bonds; therefore, they affect only slightly the reduction potentials of C60 derivatives. The potential shift reported in the literature for this class of carbon clusters is in the limits of up to 200 mV.15 Meanwhile, strong electron-withdrawing substituents in methano-16 or azahomofullerenes17 can overcompensate for the loss of electron affinity as a result of disruption of π-system of conjugation. The cyclopentane and cyclohexane derivatives of C60 are 100 and 80 mV more difficult to reduce than parent C60,18 while the presence of a nitrogen atom in fulleropyrrolidines has a slight influence on the potentials of the first two reduction steps compared with cyclopentane derivatives.19
The electrochemical reduction of fulleropyrrolidine 5 was found to occur at more negative potentials than that of the parent C60 (the difference is 60–90 mV). This distinguish compound 5 from unsubstituted pyrrolidinofullerene. This results indicate that the introduction of an aryl substituent and ester group into the heterocyclic fragment of 5 compensates, to a certain extent although not completely, for the loss of the electron affinity caused by disruption of the π-system of conjugation.
Unfortunately, we were unable to correctly calculate the EHOMO and thus to determine the band gap width of the acceptor (Eg) on the basis of cyclic voltammetry data because the oxidation waves of compound 5 are multielectron, which may be caused by sample polymerization under conditions of electrochemical measurements.
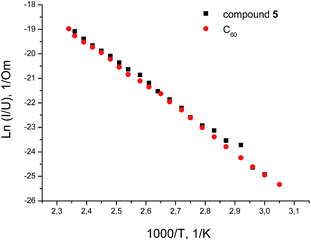
Nevertheless, the band gap width can be determined by electrophysical methods, in particularly, from the plot of the dependence of the film conductivity G on temperature T in the range of 300–450 K. These dependences are exponential, G = G0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/2kT). From the slope of the linear segments, it was possible to calculate ΔE for each sample; the result was about 1.5–1.6 eV. Meanwhile, the ΔE value can be interpreted as Eg (the gap between the LUMO and the HOMO).
exp(−ΔE/2kT). From the slope of the linear segments, it was possible to calculate ΔE for each sample; the result was about 1.5–1.6 eV. Meanwhile, the ΔE value can be interpreted as Eg (the gap between the LUMO and the HOMO).
It is found that the conductivity of 5 differs little from unmodified fullerene (Table 3), but is characterized by better solubility in organic solvents (Fig. 2).
| Compound | G, ohm−1 (at 60 °C) | ΔE, eV |
|---|---|---|
| 5 | 1.5 × 10−11 | 1.54 |
| C60 | 1.4 × 10−11 | 1.63 |
Conclusions
Thus, we developed a new method for the synthesis of N-unsubstituted pyrrolidinofullerenes by the Ti(Oi-Pr)4-catalyzed reaction of C60 with isocyanoacetates and EtMgBr. The electrochemical reduction of model pyrrolidinofullerene occurs less easily than that of C60 but more easily than that of unsubstituted pyrrolidinofullerenes, which may imply that introduction of new substituents into the heterocyclic fragment compensates somewhat for the loss of electron affinity of the fullerene cage.Acknowledgements
This work was supported by the Russian Foundation for Basic Research (Grant No. 15-03-01042), the Russian Science Foundation (Grant No. 14-13-00296) and Ministry of Education and Science of the Russian Federation (Sci.Sc.-6651.2016.3). The structural studies of fullerene derivatives were performed with use of Collective Usage Centre "Agidel" at Institute of Petrochemistry and Catalysis of RAS and "Spectro-Analytical Center" at the Institute of Organic and Physical Chemistry AE Arbuzov of RAS.Notes and references
- (a) R. Fong, D. I. Schuster and S. R. Wilson, Org. Lett., 1999, 1, 729 CrossRef CAS PubMed; (b) T. Da Ros and M. Prato, Chem. Commun., 1999, 663 RSC; (c) A. Hirsch and M. Brettreich, Fullerenes, Wiley-VCH, Weinheim, 2005, p. 426 Search PubMed.
- T. Yamashiro, Y. Aso, T. Otsubo, H. Tang, Y. Harima and K. Yamashita, Chem. Lett., 1999, 28, 443 CrossRef.
- (a) D. G. Zheng, Y. L. Li, Z. Mao and B. Zhu, Synth. Commun., 1998, 28, 879 CrossRef CAS; (b) Y.-L. Li, Z. Mao, J.-H. Xu, Y.-K. Yang, Z.-X. Guo, D.-B. Zhu, J.-W. Li and B. Yin, Chem. Phys. Lett., 1997, 361 CrossRef CAS; (c) Y.-L. Li, J.-H. Xu, D.-G. Zheng, J. K. Yang, C.-Y. Pan and D.-B. Zhu, Solid State Commun., 1997, 101, 123 CrossRef CAS.
- B. Ma, C. E. Bunker, R. Guduru, X. F. Zhang and Y. P. Sun, J. Phys. Chem. A, 1997, 101, 5626 CrossRef CAS.
- (a) A. R. Tuktarov, A. A. Khuzin, L. M. Khalilov, A. R. Tulyabaev, A. R. Akhmetov and U. M. Dzhemilev, Mendeleev Commun., 2015, 25, 470 CrossRef CAS; (b) S. Castellanos, A. A. Vieira, B. M. Illescas, V. Sacchetti, C. Schubert, J. Moreno, D. M. Guldi, S. Hecht and N. Martin, Angew. Chem., Int. Ed., 2013, 52, 13985 CrossRef CAS PubMed; (c) A. R. Tuktarov, A. A. Khuzin, A. R. Akhmetov, V. A. Barachevsky, O. V. Venidiktova and U. M. Dzhemilev, Tetrahedron Lett., 2015, 56, 7154 CrossRef CAS; (d) P. A. Liddell, G. Kodis, A. L. Moore, T. A. Moore and D. Gust, J. Am. Chem. Soc., 2002, 124, 7668 CrossRef CAS PubMed; (e) J.-H. Xu, Y.-L. Li and D.-B. Zhu, Synth. Commun., 2002, 32, 1647 CrossRef CAS; (f) J. Frey, G. Kodis, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Phys. Chem. A, 2013, 117, 607 CrossRef CAS PubMed; (g) J. Zhang, K. Porfyrakis, J. J. L. Morton, M. R. Sambrook, J. Harmer, L. Xiao, A. Ardavan and G. A. D. Briggs, J. Phys. Chem. C, 2008, 112, 2802 CrossRef CAS.
- (a) H. Imahori, H. Yamada, Y. Nishimura, I. Yamazaki and Y. Sakata, J. Phys. Chem. B, 2000, 104, 2099 CrossRef CAS; (b) H. Imahori and Y. Sakata, Eur. J. Org. Chem., 1999, 10, 2445 CrossRef; (c) P. A. Troshin, R. N. Lyubovskaya and V. F. Razumov, Nanotechnol. Russ., 2008, 3, 242 CrossRef; (d) D. Mi, H.-U. Kim, J.-H. Kim, F. Xu, S.-H. Jin and D.-H. Hwang, Synth. Met., 2012, 162, 483 CrossRef CAS.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS.
- (a) G. E. Lawson, A. Kitaygorodskiy, B. Ma, C. E. Bunker and Y.-P. Sun, Chem. Commun., 1995, 2225 RSC; (b) K.-F. Liou and C.-H. Cheng, Chem. Commun., 1996, 1423 RSC; (c) S.-H. Wu, D.-W. Zhang, G.-W. Wang, L.-H. Shu, H.-M. Wu, J.-F. Xu and X.-F. Lao, Synth. Commun., 1997, 27, 2289 CrossRef CAS; (d) S.-H. Wu, G.-W. Wang, L.-H. Shu, H.-M. Wu, S.-K. Jiang and J.-F. Xu, Synth. Commun., 1997, 27, 1415 CrossRef CAS; (e) G. E. Lawson, A. Kitaygorodskiy and Y.-P. Sun, J. Org. Chem., 1999, 64, 5913 CrossRef CAS; (f) L.-W. Gao, X. Gao, D.-W. Zhang, S.-H. Wu, H.-M. Wu and Y.-J. Li, J. Org. Chem., 2000, 65, 3804 CrossRef.
- (a) U. M. Dzhemilev, A. G. Ibragimov, M. Pudas, V. A. D'yakonov and A. R. Tuktarov, Russ. J. Org. Chem., 2007, 43, 370 CrossRef CAS; (b) O. A. Troshina, P. A. Troshin, A. S. Peregudov and R. N. Lyubovskaya, Mendeleev Commun., 2007, 17, 113 CrossRef CAS; (c) S. Filippone, E. E. Maroto, A. Martín-Domenech, M. Suarez and N. Martín, Nat. Chem., 2009, 1, 578 CrossRef CAS PubMed; (d) U. M. Dzhemilev and A. R. Tuktarov, Metal complex catalysis in the chemistry of fullerenes, in: Handbook on Fullerene: Synthesis, properties and applications, ed. R. F. Verner and C. Benvegnu, Nova Science Publishers, Inc., ISBN 978-1-62100-429-5, 2012, pp. 241–312 Search PubMed.
- (a) U. M. Dzhemilev, M. A. Famutdinova, N. R. Popod'ko and A. R. Tuktarov, Tetrahedron Lett., 2013, 54, 3260 CrossRef CAS; (b) A. R. Tuktarov, A. A. Khuzin, Z. R. Shakirova and U. M. Dzhemilev, Tetrahedron Lett., 2014, 55, 5003 CrossRef CAS; (c) A. R. Tuktarov, Z. R. Shakirova, A. A. Khuzin, A. R. Tulyabaev, I. R. Ramazanov and U. M. Dzhemilev, Synthesis, 2016, 48, 136 CrossRef CAS; (d) A. R. Tuktarov, Z. R. Shakirova, A. A. Khuzin, A. R. Tulyabaev, I. R. Ramazanov and U. M. Dzhemilev, Tetrahedron Lett., 2016, 40 DOI:10.1016/j.tetlet.2016.08.044.
- Y. Tsunenishi, H. Ishida, K. Itoh and M. Ohno, Synlett, 2000, 9, 1318 Search PubMed.
- D. Liu, S. Yang and S.-T. Lee, J. Phys. Chem. C, 2008, 112, 7110 CAS.
- T. Wakahara, Y. Maeda, M. Kako, T. Akasaka, K. Kobayashi and S. Nagase, J. Organomet. Chem., 2003, 685, 177 CrossRef CAS.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23, 2367 CrossRef CAS PubMed.
- V. V. Yanilkin, Elektrokhimiya Fullerenov (Fullerene Electrochemistry), A chapter of the collective monograph Elektrokhimiya Organicheskikh Soedinenii v Nachale XXI Veka (Electrochemistry of Organic Compounds at the Beginning of the 21st Century), ed. V. P. Gultyai, A. G. Krivenko and A. P. Tomilov, Sputnik, Moscow, 2008, pp. 178–249 Search PubMed.
- K. M. Keshavarz, B. Knight, R. C. Haddon and F. Wudl, Tetrahedron, 1996, 52, 5149 CrossRef.
- O. G. Sinyashin, I. P. Romanova, G. G. Yusupova, A. A. Nafikova, V. I. Kovalenko, N. M. Azancheev, V. V. Yanilkin and Y. G. Budnikova, Mendeleev Commun., 2000, 10, 61 CrossRef.
- T. Suzuki, Y. Maruyama, T. Akasaka, W. Ando, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 1994, 116, 1359 CrossRef CAS.
- (a) V. P. Gubskaya, E. V. Ovechkina, V. V. Yanilkin, V. I. Morozov, N. V. Nastapova, V. V. Zverev, N. M. Azancheev and I. A. Nuretdinov, Russ. Chem. Bull., 2005, 54, 334 CrossRef CAS; (b) I. A. Nuretdinov, V. V. Yanilkin, V. I. Morozov, V. P. Gubskaya, V. V. Zverev, N. V. Nastapova and G. M. Fazleeva, Russ. Chem. Bull., 2002, 51, 263 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15519g |
| This journal is © The Royal Society of Chemistry 2016 |