Well-monocrystallized LaCrO3 particles from a LaCrO4 precursor by supercritical hydrothermal technique
H.-N. Girisha,
G.-Q. Shao*a and
B. Basavalingub
aState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, Hubei 430070, China. E-mail: gqshao@whut.edu.cn; Fax: +86 27 87879468
bDepartment of Studies in Earth Science, University of Mysore, Mysore, Karnataka 570006, India
First published on 17th August 2016
Abstract
LaCrO3 based materials are currently utilized in a variety of electrical applications at high temperature. However, the morphology of LaCrO3 powder is much less understood as compared to its bulk counterpart. In this work, LaCrO3 powder was synthesized from LaCrO4 precursor by a supercritical hydrothermal technique (SHT). The majority exhibited a pure orthorhombic phase with well-monocrystallized grains of 3–9 μm. This work confirms that dominating faces in LaCrO3 grains are {0 1 0} and {0 1 1} surfaces, which have equivalent surface-energy due to the similarity of their surface structure.
1. Introduction
LaCrO3 and related materials have drawn great attention recently as high-temperature electrodes in magnetohydrodynamic power generators, oxygen sensors, oxygen transport membranes for air separation and oxy-fuel combustion. They are also used as heating elements for electric appliances and interconnects in solid oxide fuel cells.1–4 Many methods, through modifying raw materials, preparing temperature (T = 180–1600 °C), time (5 min–168 h or longer) and pressure (P = 1 atm–10.3 GPa), have been used to investigate LaCrO3. Among them the mild hydrothermal technique (MHT) is applied at 240–260 °C for 96–168 h 5,6 in an autoclave filled with aqueous solution under autogenous pressure (e.g. 260 °C/4.7 MPa). The obtained LaCrO3 powder has a morphology with a cubic habit (1–13 μm). The supercritical hydrothermal technique (SHT) has advantages over MHT to obtain LaCrO3 powder with high purity and homogeneity, fine crystallinity, narrow size distribution and controlled particle morphology. Beyond the supercritical point (373.946 °C/22.064 MPa)7 in the liquid–vapor space, water exists as small but liquid-like (associated) hydrogen-bonded clusters dispersed within a gas-like (dissociated) phase. The physical properties of the solvent such as dielectric constant (ε), density, ionic product, viscosity, self-diffusion, compressibility, etc. vary with T and/or P. The chemical properties are also greatly changed due to the changes in dissociation, solubility, diffusivity and reactivity aroused by hydrogen-bonding decreasing.8,9Yoshimura et al.10,11 prepared LaCrO3 powder via SHT using La2O3 and Cr(OH)3·xH2O as starting materials. Pure orthorhombic LaCrO3 with irregular grains (0.7 ± 0.2 μm) was obtained (700 °C/3 h/100 MPa). Their work is innovative but has obvious imperfections. The oxide of La2O3 reacts heterogeneously with the hydrate of Cr(OH)3·xH2O. The obtained grains are irregular. The hydrate can easily absorb CO2 from atmosphere, thus introducing impure LaCO3OH phase. Another work by Rivas-Vázquez et al.12,13 may be considered as a quasi-SHT using LaCl3·7H2O, Cr(NO3)3·9H2O and NaOH as starting materials, because its temperature (350–425 °C) is in the vicinity of the supercritical point. Pure orthorhombic LaCrO3 with irregular grains (∼0.3 μm) was obtained. The reaction temperature and autogenous pressure are not high enough and the time is too short (1–2 h). The precursor from the strong alkaline solution contains Na+ which is difficult to be eliminated.
Considering the supercritical water is a poor solvent for polar molecules but excellent for non-polar ones due to its low dielectric constant and poor H-bonding, many non-polar compounds could be completely miscible.8,9,14 Higher pressure than the supercritical point is beneficial to enhance the reaction rate and supersaturation during nucleation and crystallization, but it would arouse the dielectric constant increasing. Otherwise, the Cr–O bond with [CrO4] tetrahedra in LaCrO4 has a stronger covalent character than that with [CrO6] octahedra in LaCrO3, and the La–O bond in LaCrO4 is more ionic than that in LaCrO3.15 Therefore, this work is to synthesize LaCrO3 powder via SHT under the condition (700 °C/200 MPa) which is different from those just beyond the supercritical point, and using a non-hydroxide of LaCrO4 precursor as the starting material. The phase purity, crystallization, particle size distribution and morphology are highly concerned.
2. Experimental procedure
LaCrO4 precursor was synthesized by co-precipitation and subsequent calcination at 600 °C.16,17 Raw materials were analysis-graded La2O3, Cr2O3, HNO3 and ammonium solution (LOBA Chemie Co. Ltd.), and double-distilled water. Then the LaCrO4 precursor (0.19 g) was encapsulated with water (0.24 g) in a gold capsule (4 mm external dia., 40 mm long, 0.1 mm thick, ∼0.45 mL) and vacuum-sealed by arc welding. The capsule was placed in the autoclave after seal-checking by hot water. Finally, LaCrO3 powder was obtained via SHT in an externally-heated Tuttle–Roy vessel at 700 °C for 96 h under 200 MPa (H2O). Experiments were repeated (e.g. using LaCrO4 ∼0.2 g and water 0.24–0.29 g) for several times to verify the reproducibility of products.The reaction process was studied by a differential scanning calorimetric and thermo-gravimetric instrument (DSC/TG, Netzsch STA 449C, Germany) at 10 °C min−1 in N2 (20 mL min−1). Phase determination was carried out by X-ray diffraction (XRD) at room temperature (RT), using a D8 Advance diffractometer (Bruker, Germany) at 0.02° s−1 (Cu Kα, 40 kV, 40 mA). The microstructure and composition of samples were examined by a field-emission scanning electron microscope (FESEM, HITACHI S-4800, Japan) and a transmission electron microscope (TEM, JEM-2100, JEOL, Japan) with selection area electron diffraction (SAED), equipped severally with an X-ray spectrometer for energy dispersive spectroscopy (EDS) analyses.
3. Results and discussion
3.1. Reaction process from LaCrO4 precursor to LaCrO3 powder
DSC/TG profiles of the precursor and powder via SHT, coupled with differential thermal gravity (DTG) curve, are shown in Fig. 1.For the precursor, the endothermic peak at 814.7 °C, mass loss of −7.11 wt% (theoretical value −6.28 wt%) and DTG valley ranging 650–850 °C correspond to the decomposition:
 | (1) |
This is in agreement with the reported (663–840 °C).15,18,19 The endothermic peak at 1083.1 °C corresponds to the transition of LaCrO3 from rhombohedral phase to cubic one, in agreement with the reported by Gupta et al. (1000–1300 °C)20 but not with that by Hofer et al. (1550–1600 °C).21
For the powder via SHT, the endothermic peak at 240.1 °C corresponds to the transition of LaCrO3 from orthorhombic phase to rhombohedral one, in agreement with the reported (236–290 °C).5,6,12,13,19,20 The exothermic peak at 710.5 °C, mass loss of −0.35 wt% and DTG valley correspond to the crystallization of trace La-rich compounds aroused by SHT (Fig. 2 and 3, Table 1, eqn (4) and (5)). The endothermic peak corresponding to the transition of LaCrO3 from rhombohedral phase to cubic one has been merged in the wide valley. The exothermic peak at 1152.7 °C, mass increase of 1.45 wt% and DTG peak correspond to La2Cr3O12 formation from the relieved O2 and La2O3/Cr2O3 impurities which induced by the unexpected LaCrO4 decomposition:15
 | (2) |
 | ||
| Fig. 2 XRD patterns of the precursor (a) and powder via SHT (b) determined at RT. The inset is the enlargement from 30° to 80° for the powder via SHT. | ||
| EDS mapping microarea | Grain size (μm) | La/Cr (mol%) | Particles quantity |
|---|---|---|---|
| No. 1 | 0.1–0.4 | ∼1 | Trace |
| No. 2 | 0.5–1 | 1.4–1.7 | Trace |
| No. 3 | 1–3 | 6–8 | Trace |
| No. 4 | 3–9 | ∼1 | Majority |
| No. 5 | >9 | ∼1 | Trace |
3.2. Phase determination of LaCrO4 precursor and LaCrO3 powder
XRD patterns of the precursor and powder via SHT are shown in Fig. 2.The precursor resultant exhibits a pure monoclinic LaCrO4 (pdf 89-0448, CSD 81938).18,19  For the powder via SHT, the identified double peaks can tell that the powder is nearly a pure orthorhombic LaCrO3 (pdf 89-4504, CSD 50755)22 with trace impurities. Here the peaks ranging 90–140° for LaCrO3 powder were identified according to the refinement based on CSD 50755, and the refined lattice parameters are a = 5.5162(3) Å, b = 5.4802(6) Å, c = 7.7583(3) Å. The reaction can be expressed as:
For the powder via SHT, the identified double peaks can tell that the powder is nearly a pure orthorhombic LaCrO3 (pdf 89-4504, CSD 50755)22 with trace impurities. Here the peaks ranging 90–140° for LaCrO3 powder were identified according to the refinement based on CSD 50755, and the refined lattice parameters are a = 5.5162(3) Å, b = 5.4802(6) Å, c = 7.7583(3) Å. The reaction can be expressed as:
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO2 and ΔG0 = 85731 − 85.096T.15 When ΔG = 0, the LaCrO4 starts to decompose: ln
PO2 and ΔG0 = 85731 − 85.096T.15 When ΔG = 0, the LaCrO4 starts to decompose: ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO2 = −20
PO2 = −20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 623/T + 20.471. Initially these equations satisfied an open system, while this work involved a closed one. Considering that supercritical water is completely miscible with non-polar oxygen,14,23 the above equations could then be imported in our system. At the supercritical point of 373.946 °C, PO2 is equal to 10−5 atm. At the process temperature of 700 °C, PO2 is 0.48 atm. These indicate the SHT is a favorably thermodynamic system. If it reacted completely, the oxygen partial pressure built up inside the capsule (0.45 mL) would arrive at ∼6.7 MPa by the decomposition of precursor (0.19 g) at 700 °C. So the water pressure would be under ∼193.3 MPa, which remains all the same at the supercritical state. Otherwise, the formation of tiny impurity phases of La2CrO6 and Cr2O3 etc. (Fig. 2 and 3, Table 1, eqn (4) and (5)) would relieve the oxygen pressure. During the decomposition of LaCrO4 to LaCrO3, oxygen coordination around La atom(s) changes from 9 to 12 and around Cr atom(s) from 4 to 6. The major structural rearrangement during decomposition is thus mainly linked to high activation energy (∼18 kJ mol−1)24 and slow kinetics.15
623/T + 20.471. Initially these equations satisfied an open system, while this work involved a closed one. Considering that supercritical water is completely miscible with non-polar oxygen,14,23 the above equations could then be imported in our system. At the supercritical point of 373.946 °C, PO2 is equal to 10−5 atm. At the process temperature of 700 °C, PO2 is 0.48 atm. These indicate the SHT is a favorably thermodynamic system. If it reacted completely, the oxygen partial pressure built up inside the capsule (0.45 mL) would arrive at ∼6.7 MPa by the decomposition of precursor (0.19 g) at 700 °C. So the water pressure would be under ∼193.3 MPa, which remains all the same at the supercritical state. Otherwise, the formation of tiny impurity phases of La2CrO6 and Cr2O3 etc. (Fig. 2 and 3, Table 1, eqn (4) and (5)) would relieve the oxygen pressure. During the decomposition of LaCrO4 to LaCrO3, oxygen coordination around La atom(s) changes from 9 to 12 and around Cr atom(s) from 4 to 6. The major structural rearrangement during decomposition is thus mainly linked to high activation energy (∼18 kJ mol−1)24 and slow kinetics.15
3.3. Microstructure and chemical composition of LaCrO3 powder via SHT
Fig. 3 and Table 1 list the SEM/EDS results of the LaCrO3 powder. Five kinds of particles are classified based on grain sizes through EDS mapping in the microarea No. 1 to No. 5, respectively. The majority of particles are pure (La/Cr ≈ 1) and well-crystallized grains (3–9 μm, No. 4). The others have only a trace quantity. Most grains have facetted morphology with {0 1 0} and {0 1 1} as dominating faces. A few of them have rectangular prismatic morphology with {0 0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) }, {1
}, {1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} and {
0} and {![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} (Fig. 3a). This is different with the reported10–13 in which irregular particles attained by SHT. Through ordinary MHT, LaCrO3 particles are with a cubic habit.5,6 Our work confirms first that dominating faces in LaCrO3 grains are {0 1 0} and {0 1 1} which have equivalent surface-energy due to the similarity of their surface structure.25 Such morphology could previously only be found in sintered samples through high temperature (e.g. 1450 °C/10 h/air),20 indicating a drastically-increasing sinterability through SHT process.
0} (Fig. 3a). This is different with the reported10–13 in which irregular particles attained by SHT. Through ordinary MHT, LaCrO3 particles are with a cubic habit.5,6 Our work confirms first that dominating faces in LaCrO3 grains are {0 1 0} and {0 1 1} which have equivalent surface-energy due to the similarity of their surface structure.25 Such morphology could previously only be found in sintered samples through high temperature (e.g. 1450 °C/10 h/air),20 indicating a drastically-increasing sinterability through SHT process.
Particles with small (0.1–0.4 μm, No. 1) or very large (>9 μm, No. 5) sizes are also pure LaCrO3 (La/Cr ≈ 1). Two kinds of La-rich particles were found. One has a grain size of 0.5–1 μm with a La/Cr ratio of 1.4–1.7 (No. 2). The other is 1–3 μm with a La/Cr ratio of 6–8 (No. 3). The crystallite formation, growth and crystallization, from the LaCrO4 precursor to LaCrO3 grains, are initially as:15
 | (4) |
Being more amphoteric, the Cr3+-hydroxide condenses less readily than the La3+-hydroxide at a given alkalinity.6 The oxidation product of tiny CrO42− ions could be dissolved in the supercritical water and washed out later. This explains the large La/Cr ratio of 1.4–1.7 in La-rich particles.
Next, La2CrO6 decomposes to a mixture of LaCrO3 and La2O3:15
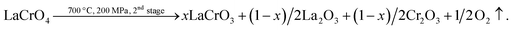 | (5) |
Same analysis in eqn (4) can be applied in eqn (5). The large La/Cr ratio of 6–8 is due to the La2O3 resultant. The crystallization of one oxide in ordinary MHT proceeds by a dissolution–crystallization mechanism. However, the crystallization of LaCrO3 in SHT proceeds simultaneously with the decomposition of LaCrO4.12,13 The supercritical water gives a favorable reaction field for particle formation, owing to the enhancement of reaction rate and large supersaturation during nucleation and crystallization through lowering the solubility of LaCrO3 resultant. The process lasted 96 h and the reaction equilibrium is sufficient.
TEM images and SAED patterns of two typical LaCrO3 grains (a and b) are shown in Fig. 4. The dark contrast is aroused by large size of grains (∼5 μm for Grain-a in Fig. 4a-I, and ∼9 μm for Grain-b in Fig. 4b-I). Both grains have a La/Cr ratio close to 1 by EDS (not show here). From the SAED pattern of Grain-a (Fig. 4a-II), it can only be indexed as [0 0 1] zone axis of the orthorhombic LaCrO3. By tilting 18.85°, the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3] zone axis was obtained (Fig. 4a-III). For Grain-b, the [
3] zone axis was obtained (Fig. 4a-III). For Grain-b, the [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2] (Fig. 4b-II) and [0
2] (Fig. 4b-II) and [0 ![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3] (Fig. 4b-III) zone axes with tilting degree of 19.7° can also be indexed to the orthorhombic phase. These SAED analyses have unambiguously confirmed the crystal symmetry of orthorhombic structure in LaCrO3 samples by SHT. No cubic LaCrO3 particles were observed. LaCrO3 particles with single crystallites give a promising prospect for better properties comparing to those with polycrystallites.
3] (Fig. 4b-III) zone axes with tilting degree of 19.7° can also be indexed to the orthorhombic phase. These SAED analyses have unambiguously confirmed the crystal symmetry of orthorhombic structure in LaCrO3 samples by SHT. No cubic LaCrO3 particles were observed. LaCrO3 particles with single crystallites give a promising prospect for better properties comparing to those with polycrystallites.
4. Conclusions
In this work, nearly pure orthorhombic LaCrO3 particles, with well-monocrystallized grains of 3–9 μm, were synthesized at 700 °C for 96 h under 200 MPa by supercritical hydrothermal technique. The results confirm first that most of the obtained LaCrO3 grains have facetted morphology with {0 1 0} and {0 1 1} as dominating faces. Such morphology could previously only be found in the sintered samples through high temperature, indicating a drastically-increasing sinterability through this process.Acknowledgements
Authors gratefully thank Mr J.-H. Liu, Mr Z.-S. Gao, Mr D. Chen, Dr J. Li, Ms G.-G. Zhao and Ms H.-F. Zhang in WUT, Prof. B.-L. Wu in GUT, and Prof. R. Huang in ECNU for his TEM analysis. This work was supported by the Postdoctoral Foundation of WUT (Grant No. G2014-2016), and the Research Foundation of SKLWUT, China (Grant No. 2014-KF-6, 2015-KF-4 & 2016-KF-4).References
- S. Gupta, M. K. Mahapatra and P. Singh, Mater. Sci. Eng., R, 2015, 90, 1 CrossRef.
- B. Tiwari, A. Dixit, R. Naik, G. Lawes and M. S. R. Rao, Mater. Res. Express, 2015, 2, 026103 CrossRef.
- Y.-P. Chen, H.-W. Qin, C.-M. Shi, L. Li and J.-F. Hu, RSC Adv., 2015, 5, 54710 RSC.
- H. Bhatt, J. Bahadur, M. N. Deo, S. Ramanathan, K. K. Pandey, D. Sen, S. Mazumder and S. M. Sharma, J. Solid State Chem., 2011, 184, 204 CrossRef CAS.
- W. Hu, Y. Chen, H. Yuan, G. Zhang, G. Li, G. Pang and S. Feng, J. Solid State Chem., 2010, 183, 1582 CrossRef CAS.
- W. Zheng, W. Pang, G. Meng and D. Peng, J. Mater. Chem., 1999, 9, 2833 RSC.
- B. Rukes and R. B. Dooley, Guideline on the Use of Fundamental Physical Constants and Basic Constants of Water, International Association for the Properties of Water and Steam, Gaithersburg, Maryland, USA, 2001, p. 1 Search PubMed.
- T. Adschiri, Y. Hakuta and K. Arai, Ind. Eng. Chem. Res., 2000, 39, 4901 CrossRef CAS.
- M. Chaplin, Water Structure and Science, http://www1.lsbu.ac.uk/water/index.html/, accessed Oct. 23, 2015.
- M. Yoshimura, S.-T. Song and S. Sōmiya, J. Ceram. Soc. Jpn., 1982, 90, 91 CrossRef CAS.
- M. Yoshimura, S.-T. Song and S. Sōmiya, Ferrites: Proc. Int'l Conf., ed. H. Watanabe, S. Iida and M. Sugimoto, Japan, 1980, p. 429 Search PubMed.
- L. P. Rivas-Vázquez, J. C. Rendón-Angeles, J. L. Rodríguez-Galicia, C. A. Gutiérrez-Chavarria, K. J. Zhu and K. Yanagisawa, J. Eur. Ceram. Soc., 2006, 26, 81 CrossRef.
- L. P. Rivas-Vázquez, J. C. Rendón-Angeles, J. L. Rodriguez-Galicia, K. Zhu and K. Yanagisawa, Solid State Ionics, 2004, 172, 389 CrossRef.
- H. Weingärtner and E. U. Franck, Angew. Chem., Int. Ed., 2005, 44, 2672 CrossRef PubMed.
- T. J. Kallarackel, S. Gupta and P. Singh, J. Am. Ceram. Soc., 2013, 96, 3933 CrossRef CAS.
- B. Basavalingu, H. N. Girish, K. Byrappa and K. Soga, Mater. Chem. Phys., 2008, 112, 723 CrossRef CAS.
- H. N. Girish, B. Basavalingu, G.-Q. Shao, C. P. Sajan and S. K. Verma, Mater. Sci.–Pol., 2015, 33, 301 Search PubMed.
- J. D. Carter, H. U. Anderson and M. G. Shumsky, J. Mater. Sci., 1996, 31, 551 CrossRef CAS.
- S. Bilger, G. Blaf and R. Forthmann, J. Eur. Ceram. Soc., 1997, 17, 1027 CrossRef CAS.
- S. Gupta, M. K. Mahapatra and P. Singh, Mater. Res. Bull., 2013, 48, 3262 CrossRef CAS.
- H. E. Hofer and W. F. Kock, J. Electrochem. Soc., 1993, 140, 2889 CrossRef.
- R. H. Mitchell and A. R. Chakhmouradian, J. Solid State Chem., 1999, 144, 81 CrossRef CAS.
- M. L. Japas and E. U. Franck, Ber. Bunsen-Ges., 1985, 89, 1268 CrossRef CAS.
- Y. Aoki, H. Konno and H. Tachikawa, J. Mater. Chem., 2001, 11, 1214 RSC.
- A. Kelly and K. M. Knowles, Crystallography and Crystal Defects, John Wiley & Sons, Ltd, UK, 2nd edn, 2012, p. 391 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |



