Superhydrophobic magnetic nanoparticle-free fatty acid regenerated from waste cooking oil for the enrichment of carcinogenic polycyclic aromatic hydrocarbons in sewage sludges and landfill leachates
Siti Khalijah Mahmad Roziab,
Shabnam Bakhshaeia,
Ninie Suhana Abdul Manana and
Sharifah Mohamad*a
aDepartment of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: sharifahm@um.edu.my; Fax: +60-379674193; Tel: +60-379676751
bSchool of Bioprocess Engineering, University of Malaysia Perlis, Kompleks Pusat Pengajian Jejawi 3, 02600 Arau, Perlis, Malaysia
First published on 29th August 2016
Abstract
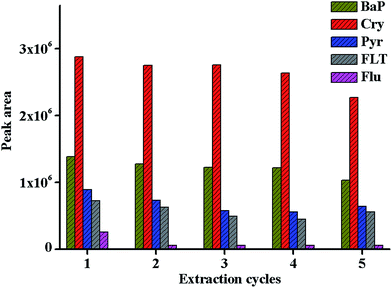
In this study, Fe3O4 nanoparticles grafted with superhydrophobic free fatty acids from waste cooking oil (FFA@MNP) were successfully fabricated as an adsorbent for the magnetic solid phase extraction (MSPE) technique. The synthesized nanomaterial (FFA@MNP) was analyzed using Fourier transform infrared spectroscopy, transmission electron microscopy, X-ray diffraction, energy dispersive X-ray spectroscopy, water contact angle analysis and vibrating sample magnetometry, which confirmed the successful functionalization of free fatty acids onto the surface of Fe3O4 nanoparticles. The adsorption efficiency of the synthesised FFA@MNP in MSPE was evaluated by the extraction of five selected polycyclic aromatic hydrocarbons (PAHs), namely, fluorene (Flu), fluoranthene (FLT), pyrene (Pyr), chrysene (Cry), and benzo(a)pyrene (BaP), from environmental samples prior to HPLC-DAD analysis. The MSPE method was optimized for various parameters such as adsorbent dosage, type of organic eluent, volume of organic eluent, extraction time, desorption time, pH of the solution and sample volume. The low detection limit for the proposed MSPE was found to be 0.001 to 0.05 ng mL−1. Under optimized conditions, the newly synthesized material was applied as an adsorbent for environmental leachate and sludge samples for enrichment of the selected PAHs from these complex matrices. The stability and reusability studies demonstrated that FFA@MNP could be used for up to five cycles. Due to hydrophobic interactions between the long alkyl chains of FFA@MNP and PAHs, good recoveries (82.8–116.6%) with low relative standard deviations ranging from 5.2% to 11.0% were obtained for the spiked leachate samples. For the spiked sludge samples, the recovery range was found to be from 72.3% to 119.9%, with a satisfactory % RSD (4.8–11.8%).
1. Introduction
The chemical contamination of our environment over the past few decades as a result of hazardous pollutants, such as polycyclic aromatic hydrocarbons (PAHs), is becoming a serious threat owing to the carcinogenic and mutagenic nature of these pollutants. PAHs belongs to the class of potential pollutants which are listed as priority pollutants by the United States Environmental Protection Agency.1,2 These PAHs accumulate in our environment due to anthropogenic activities such as combustion of fossil fuel and industrial pollution.3 Due to their unique chemical structures, consisting of many aromatic rings, PAHs are considered to be environmentally persistent organic compounds which may pose dangers to our environment and to living beings.4Polycyclic aromatic hydrocarbons (PAHs) are one of the most common classes of toxic organic pollutants, they are usually detected in landfills. They are released and formed in these areas mainly via petroleum hydrocarbon residues and direct burning of landfills. Therefore, PAHs have been frequently detected in landfill leachates,5,6 with concentrations of up to 7966 μg L−1 depending on the variety of their composition and the degree of industrial pollution.7 Moreover, due to the high lipophilicity and low biodegradability of PAHs in our environment, they are also found in sewage sludge.8–10 The detection of PAHs in sewage sludge has become an important issue in Europe, as the maximum level of PAHs allowed in sewage sludge is 6000 ng g−1 (calculated as the sum of acenapthene, phenanthrene, fluorene, fluoranthene, pyrene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[g,h,i]perylene and indeno[1,2,3-cd]pyrene) as released by the 3rd draft directive of the European Union. In addition, PAHs in landfills from pyrolytic processes are complex mixtures of non-substituted compounds which can be considered to be ecologically toxic chemicals. Considering these problems, there is a need for a rapid and low-cost analytical method for the detection of PAHs in leachate and sludge samples.
As PAHs are present in leachate and sludge samples at trace levels, a pre-concentration step is required to isolate and enrich this pollutant from matrices before instrumental analysis. Recently, a greener approach for sample pretreatment, known as magnetic solid phase extraction (MSPE), was introduced to enrich different analytes, such as phenol,11,12 phthalate ester,13,14 organophosphorus pesticides15,16 and PAHs.17,18 MSPE provides high extraction efficiency, rapid extraction kinetics, a high enrichment factor and high breakthrough volume compared to conventional extraction methods.19 This method is based on the use of a magnetizable adsorbent,20,21 which is usually based on magnetite (Fe3O4) nanoparticles (MNPs). Owing to the superparamagnetic properties of MNPs,22,23 the magnetic nano-sorbents can be simply collected with an external magnetic field, which enhances the effectiveness of the pre-treatment procedure. Because of their unique features, MNPs have been widely applied in water purification.19 Additionally, MNPs have high surface areas, lower toxicity,24,25 and facile synthesis processes. Thus, they are suitable candidates as solid sorbents for the extraction of hazardous environmental pollutants. However, the defects of MNPs, including instability in acidic environments, agglomeration in aqueous media, ready oxidation upon exposure to air and poor extraction efficiency due to their highly hydrophilic nature,26,27 limit their use as adsorbents for the extraction of organic pollutants from environmental samples. Thus, an alternative solution can be achieved by developing newly modified MNPs with intense hydrophobizing agents based on economic and eco-friendly natural biomaterials.
Herein, we highlight the facile synthesis of a new sorbent based on a green approach, namely, the impregnation of magnetite (Fe3O4) nanoparticle MNPs with biomaterials. Here, magnetic nanoparticles functionalized with free fatty acids from waste cooking oil (FFA@MNP) were used as an MSPE adsorbent for the extraction of PAHs from environmental samples. High amounts of free fatty acids can be produced in waste cooking oil through a hydrolytic process that occurs in the presence of oil and water from food. The chemical structure of free fatty acids, which consist of long alkyl chains, makes them an excellent hydrophobizing agent to tune the hydrophilic surface of MNPs for selective extraction of PAHs from complex matrices. Waste cooking oil was selected because it is easily available and economic. In addition, the utilization of a hazardous pollutant, waste cooking oil, for the determination of PAHs (Fig. 1) from the environment is a good solution for waste management. Thus, we introduce a new MSPE adsorbent, FFA@MNP, which combines high selectivity and environmental stability, for the extraction of PAHs from polluted environments.
2. Experimental section
2.1 Reagents and chemicals
N,N-dimethylformamide (DMF), (3-aminopropyl)triethoxysilane (APTES), aqueous ammonia, ethanol, choloroform, diethyl ether, methanol, acetonitrile, hexane and ethyl acetate were obtained from Merck. Ferrous chloride tetrahydrate, ferric chloride hexahydrate, potassium iodide, iodine monochloride, starch, potassium hydroxide and sodium thiosulphate were purchased from Sigma Aldrich. Flu, FLT, Pyr, Cry and BaP were purchased from Supelco. The standard stock solutions (100 mg L−1) were prepared in methanol and stored in a dark amber glass at 4 °C to prevent degradation. The working solutions were prepared daily by diluting the stock solution with deionized water. Water was deionized prior to use.The waste cooking oil used in this study was collected from a restaurant in the city of Bangsar, Kuala Lumpur. The collected waste cooking oil was used in the restaurant for frying purposes at about 180 °C for 2 days. Before use, the waste cooking oil samples were filtered in order to eliminate particulate materials and other impurities. This waste cooking oil was characterized by its acid and iodine values (Table 1). In addition, the fatty acid profile of the oil was analysed, as shown in Table 2. The gas–liquid chromatography reference standard for fatty acid methyl esters was purchased from Supelco.
| Property | Unit | Value |
|---|---|---|
| Acid value | mg KOH g−1 | 8.74 |
| Iodine value | g × 102 g−1 | 7.31 |
| Fatty acid | Fatty acid composition (%) | |
|---|---|---|
| Palmitic | (C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
40.56 |
| Stearic | (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
5.46 |
| Oleic | (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
41.73 |
| Linoleic | (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
12.26 |
2.2 Instrumentation
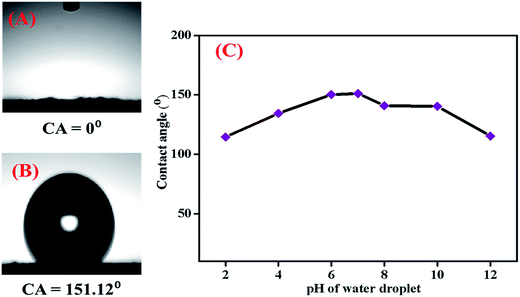
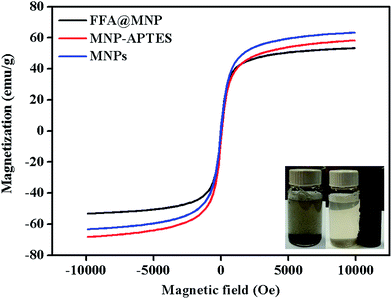
The particle size and morphology were investigated using a JEOL JEM-2100F transmission electron microscope (TEM). EDX analysis was performed using a scanning electronic microscope (SEM HITACHI SU8220, OXFORD Instruments) equipped with an energy dispersive X-ray spectrometer. FT-IR spectrometry (Spectrum 400 PerkinElmer) was performed using the ATR technique in absorption mode with 4 scans, a resolution of ±4 cm−1, and a wavenumber range of 4000 to 450 cm−1 with a diamond detector. X-ray diffraction (XRD) patterns were recorded using an Empyrean X-ray diffractometer from 2θ = 15° to 75° using Cu Kα radiation (λ = 1.5418 Å) at a scan rate of 0.02 s−1. Contact angle (CA) measurements were conducted using a contact angle instrument (TL 100 and TL 101). The magnetizations of the bare and functionalized magnetic nanoparticles were measured using a vibrating sample magnetometer (VSM LakeShore 7400 series). The acid (AV) and iodine (IV) values of the waste cooking oil were determined according to the standard method in the PORIM Test Methods.28 The fatty acid methyl esters from waste cooking oil were prepared using an acid catalysed (sulphuric acid) method.29 The analysis of the fatty acid composition of the waste cooking oil was performed using a Shimadzu 2010 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a split/splitless injector and a flame ionization detector (FID). A BPX50 capillary column (SGE Analytical Science, Australia) (30 m × 0.25 mm i.d. 0.25 μm film thickness) was applied for the separation of fatty acids. Helium (with 99.999% purity) was used as the carrier gas at a constant flow rate of 59.2 mL min−1. The chromatographic conditions were controlled as follows: the temperature of the detector and injector were set at 260 and 139 °C, respectively. The injection port was operated in split mode. The oven temperature was initially set at 139 °C for 2 min, then increased by 8 °C min−1 to 165 °C, by 3 °C min−1 to 192 °C, by 13.7 °C min−1 to 240 °C; finally, the temperature was maintained at 240 °C for 11 min.2.3 HPLC analysis
An HPLC system consisting of a Shimadzu (Tokyo, Japan) LC-20AT pump, an SPD-M20A diode array detector, an SIL-20A HT auto sampler and a CTO-10AS VP column oven was used for the separation and quantification of PAHs. The separation was conducted on a C-18 reverse column (250 mm × 4.6 mm; particle size 5 μm; Hypersil Gold, Thermo Science USA). A mobile phase of acetonitrile–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) was used at a flow rate of 1.0 mL min−1. The injection volume was 10 μL. The detection of each PAH was set as follows: 5.54 min, 254 nm (for Flu); 6.77 min, 284 nm (for FLT); 7.22 min, 270 nm (for Pyr); 8.03 min, 266 nm (for Cry); 10.82 min, 266 nm (for BaP).
20, v/v) was used at a flow rate of 1.0 mL min−1. The injection volume was 10 μL. The detection of each PAH was set as follows: 5.54 min, 254 nm (for Flu); 6.77 min, 284 nm (for FLT); 7.22 min, 270 nm (for Pyr); 8.03 min, 266 nm (for Cry); 10.82 min, 266 nm (for BaP).
2.4 Synthesis of free fatty acid functionalized Fe3O4 nanoparticles (FFA@MNP)
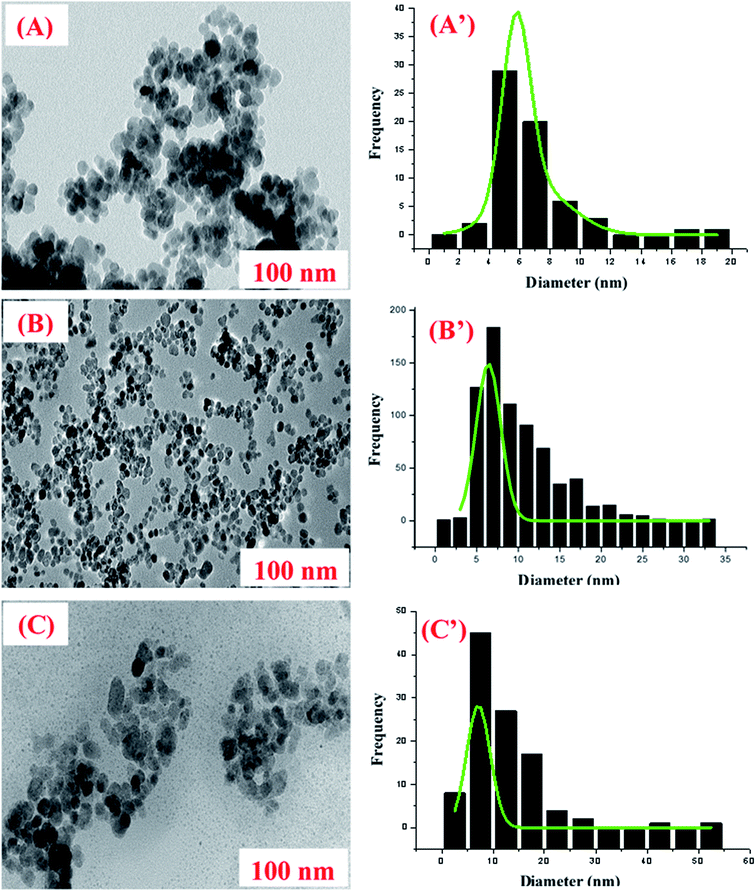
Fig. 2 depicts the procedures for the preparation of FFA@MNP. The magnetite nanoparticles (MNPs) were prepared via the chemical co-precipitation method.30 Based on this method, FeCl2·4H20 (3.1736 g) and FeCl3·6H2O (7.5709 g) were dissolved in 320 mL deionized water. The mixed solution was stirred under nitrogen at 80 °C for 1 hour. Then, 40 mL of aqueous ammonia was rapidly added to the reaction mixture; the mixture was stirred under nitrogen for another 1 hour and then cooled to room temperature. The obtained nanoparticles were magnetically collected and washed five times with hot water until the filtrate became neutral.MNP-APTES was synthesized according to the reported method.31 Briefly, the hydrolysed APTES was added to 100 mL of the magnetite fluids in a 250 mL round bottom flask. The mixture was stirred for 5 hours at 60 °C under nitrogen protection. Later, the solution was cooled at room temperature and the product was collected using filtration, then washed with ethanol and deionized water several times, respectively. The obtained product was dried under vacuum at 70 °C.
To synthesize FFA@MNP, 1.000 g of MNP-APTES was dispersed in 10 mL of DMF, followed by the addition of 2.000 g of waste cooking oil to the solution; the mixture was stirred overnight at 60 °C under nitrogen protection. After the solution was cooled to room temperature, the resulting brownish product was washed with ethanol and deionized water several times, respectively, by magnetic decantation. Then, the final product was dried under vacuum at 70 °C for 24 hours.
2.5 MSPE procedure
Firstly, 10 mg of FFA@MNP were placed in a 15 mL water sample containing trace levels of PAHs. The solution was shaken for 10 minutes to facilitate the adsorption of PAHs onto FFA@MNP (Fig. 3). Subsequently, a Nd–Fe–B magnet was applied to isolate the FFA@MNP from the sample solution. After the solution became clear, the supernatant was removed. The PAHs adsorbed on FFA@MNP were eluted with 1.5 mL of organic eluent (acetonitrile) by shaking for 10 minutes. The collected eluate was dried under a stream of N2 and then re-dissolved in 0.5 mL of acetonitrile. Finally, a 10 μL portion of the eluate was injected into the HPLC system for analysis. | ||
| Fig. 3 Illustration of adsorption of the targeted PAHs on the hydrophobic frameworks of FFA@MNP during the MSPE procedure. (R = the alkyl chains of the free fatty acids). | ||
2.6 Real sample preparation
All the samples, including leachate and sludge, were collected from a landfill at Jeram, Kuala Selangor. The leachate samples were filtered through a 0.22 μm cellulose membrane immediately after sampling and were stored at 4 °C before analysis. Then, 150 mL of filtered leachate sample was used for MSPE by the method mentioned above.For the sludge sample, the collected sample was dried at room temperature, ground and sieved. The extraction of soil was carried out by ultrasound-assisted extraction (UAE) using the following procedure: 1 g of the sludge sample was exactly weighed and placed in a 50 mL centrifuge tube; 3 mL of methanol was added, and the mixture was sonicated for 10 min. After centrifugation at 3500 rpm for 5 min, the supernatant was filtered using a PTFE syringe filter (13 mm, 0.22 μm pore size) and was transferred into the sample vial.32 Subsequently, the total volume was made up to 150 mL with distilled water, and the samples were analysed by MSPE as described previously.
3. Results and discussion
3.1 Characterizations of the FFA@MNP
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at ∼1712 cm−1 and the NHCO group at ∼1405 cm−1 were observed. Furthermore, the appearance of new peaks at ∼2925 and ∼2860 cm−1 can be related to the asymmetric and symmetric methylene and methyl vibrations, respectively. These peaks confirmed the presence of the free fatty acids, which were functionalized successfully onto the surface of MNP-APTES.
O group at ∼1712 cm−1 and the NHCO group at ∼1405 cm−1 were observed. Furthermore, the appearance of new peaks at ∼2925 and ∼2860 cm−1 can be related to the asymmetric and symmetric methylene and methyl vibrations, respectively. These peaks confirmed the presence of the free fatty acids, which were functionalized successfully onto the surface of MNP-APTES.
3.2 Optimization of MSPE conditions
To achieve better extraction efficiency of the tested PAHs using FFA@MNP, several parameters, such as the adsorbent dosage, type of organic eluent, volume of organic eluent, extraction time, desorption time, pH of the solution and sample volume, were optimized. Each experiment was performed in triplicate. The experimental conditions for MSPE using the prepared FFA@MNP were herein optimized using a standard aqueous solution spiked with 100 ng mL−1. Chromatography of the peak area was used to evaluate the influence of these factors on the extraction efficiency of the MSPE of all the tested PAHs.3.3 Analytical performance
Under the optimized conditions, a series of experiments with regard to the linearity, limit of detection, limit of quantification and precision were evaluated to validate the developed method. The results are listed in Table 3. It can be seen from the table that the present method has a wide linear range and good method precision. In this case, all the tested PAHs exhibited good linearity, with correlation coefficients ranging from 0.9955 to 0.9998. The LOD and LOQ values of the PAHs were found to be in the ranges from 0.001 to 0.05 ng mL−1 and from 0.004 to 0.2 ng mL−1, respectively. Precision, expressed as relative standard deviation (RSD), was assessed in terms of repeatability (from five independent sample preparations, intra-day RSD) and reproducibility (studied during three consecutive days, inter day RSD); the values for intra-day RSD% were between 2.9 and 6.5%, and those of inter-day RSD% were in the range of 1.5–2.5%.| Analyte | Linearity | R2 | LOD (ng mL−1) | LOQ (ng mL−1) | Precision | |
|---|---|---|---|---|---|---|
| LDR (ng mL−1) | Intra-day (RSD%, n = 5) | Inter-day (RSD%, n = 3) | ||||
| Flu | 0.1–100 | 0.9996 | 0.05 | 0.2 | 6.4 | 2.2 |
| FLT | 0.01–100 | 0.9955 | 0.009 | 0.03 | 6.5 | 1.5 |
| Pyr | 0.01–100 | 0.9987 | 0.008 | 0.03 | 3.1 | 2.5 |
| Cry | 0.01–50 | 0.9998 | 0.003 | 0.009 | 5.3 | 2.3 |
| BaP | 0.01–100 | 0.9977 | 0.001 | 0.004 | 2.9 | 2.1 |
3.4 Application to real samples
To demonstrate the applicability and reliability of the proposed method, it was applied to some real samples, including leachate and sludge collected from Jeram Landfill, Kuala Selangor. To test the accuracy of the method, the recoveries of the PAHs from leachate and sludge at spiking levels of 0.5 ng mL−1, 5 ng mL−1, and 50 ng mL−1 were measured. The obtained results are shown in Table 4. It can be seen that the relative recoveries for all tested PAHs in leachate were in the range of 82.8–116.6%, with RSDs (n = 5) ranging from 5.2% to 11.0%; this implies good performance of the presented method for the determination of PAHs in real samples.| Analyte | Spiked (ng mL−1) | Leachate (n = 5) | Sludge (n = 5) | ||
|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||
| Flu | 0.5 | 111.5 | 7.5 | 112.7 | 9.6 |
| 5 | 94.0 | 9.7 | 98.3 | 9.6 | |
| 50 | 107.1 | 6.5 | 87.8 | 5.5 | |
| FLT | 0.5 | 112.1 | 6.7 | 112.7 | 9.6 |
| 5 | 116.6 | 11.0 | 103.6 | 8.6 | |
| 50 | 105.2 | 8.2 | 101.0 | 8.6 | |
| Pyr | 0.5 | 115.3 | 6.0 | 117.6 | 4.8 |
| 5 | 94.7 | 10.1 | 99.1 | 11.7 | |
| 50 | 82.8 | 6.1 | 95.1 | 9.9 | |
| Cry | 0.5 | 100.4 | 7.6 | 116.7 | 11.2 |
| 5 | 99.2 | 9.5 | 119.9 | 11.8 | |
| 50 | 105.8 | 6.7 | 107.2 | 11.7 | |
| BaP | 0.5 | 100.0 | 9.0 | 116.4 | 11.7 |
| 5 | 97.6 | 7.6 | 72.3 | 8.9 | |
| 0.5 | 110.6 | 5.2 | 73.4 | 7.9 | |
Moreover, the developed method was used for determination of the tested PAHs in sludge samples. As is obvious from Table 4, the recoveries for the spiked sludge were found to be in the range of 72.3–119.9%, with RSDs (n = 5) ranging from 4.8% to 11.8%. These results imply that the established method can be applied even for the analysis of PAHs at trace levels in complex matrices. Fig. 11 illustrates typical HPLC-DAD chromatograms of the PAHs extracted from leachate by the proposed procedure, before and after spiking with 50 ng mL−1 of each PAH.
3.5 Comparison of FFA@MNP with other reported MSPE adsorbents
The extraction efficiencies of FFA@MNP adsorbent for the enrichment of PAHs were compared with other MSPE adsorbents reported in the literature (as shown in Table 5). Comparison of the % Recovery, % RSD and LOD reveals that FFA@MNP is an efficient MSPE adsorbent compared to previously reported MSPE adsorbents.| Matrix | Technique/adsorbent | % recovery | % RSD | LODs (ng mL−1) | Ref. |
|---|---|---|---|---|---|
| Aqueous samples | MSPE-GC/magnetic C18 microspheres | 35.0 to 99.0 | <10.0 | 0.8 to 36 | 37 |
| Lake water | MSPE-HPLC/Fe3O4–SiO2-MIL-101 | 81.3 to 98.7 | 3.0 to 10.8 | 0.0028 to 0.027 | 38 |
| Lake water | MSPE-GC/Fe3O4–octadecylphosphonic acid | 53.5 to 103.5 | 0.8 to 7.6 | 0.014 to 0.0644 | 39 |
| Tap–river–seawater | MSPE-HPLC/Fe3O4–graphene oxide | 76.8 to 103.2 | 1.7 to 11.7 | 0.09 to 0.19 | 40 |
| Tap–well–river water | MSPE-GC/Fe3O4–CNFs | 93.7 to 100.9 | 3.2 to 9.8 | 0.008 to 0.03 | 41 |
| Leachate–sludge | MSPE-HPLC/Fe3O4–CN/IL | 89.50 to 110.2 | 1.2 to 4.5 | 0.86 to 1.95 | 42 |
| Leachate–sludge | MSPE-HPLC/FFA@MNP | 72.3 to 119.9 | 4.8 to 11.8 | 0.001 to 0.05 | This work |
4. Conclusion
In this research, superhydrophobic Fe3O4 nanoparticles with free fatty acids from waste cooking oil (FFA@MNP) were successfully synthesized and applied for the enrichment of trace level PAHs from leachate and sludge samples. The obtained detection limits of Flu, FLT, Pyr, Cry and BaP were in the range of 0.001 to 0.05 ng mL−1, and good recoveries were obtained in ranges of 72.3–119.9% and 82.8–116.6% for spiked sludge and leachate samples, respectively. Moreover, the novel FFA@MNP adsorbent exhibited good reproducibility and environmental stability for the detection of trace PAHs from environmental samples. With these remarkable features, FFA@MNP shows great potential as an MSPE adsorbent for the enrichment and analysis of trace hydrophobic organic pollutants in environmental samples.Acknowledgements
The authors would like to take this chance to express their appreciation to the University of Malaya for a research grant (RP020A-16SUS) and IPPP grant PG042-2015A. The authors also gratefully acknowledge the School of Bioprocess Engineering, University of Malaysia Perlis for providing a fellowship to the one of the authors, Siti Khalijah Binti Mahmad Rozi. The authors thank Dr Cheng Sit Foon and Miss Kok Wai Ming for their help with fatty acid analysis.References
- S. a. Wise, L. C. Sander and M. M. Schantz, Polycyclic Aromat. Compd., 2015, 35, 187–247 CrossRef CAS.
- H. Zhou, C. Wu, A. Meng, Y. Zhang and P. T. Williams, J. Anal. Appl. Pyrolysis, 2014, 110, 264–269 CrossRef CAS.
- L. Zhao, H. Hou, Y. Shangguan, B. Cheng, Y. Xu, R. Zhao, Y. Zhang, X. Hua, X. Huo and X. Zhao, Ecotoxicol. Environ. Saf., 2014, 108, 120–128 CrossRef CAS PubMed.
- Y. Long, Y. Chen, F. Yang, C. Chen, D. Pan, Q. Cai and S. Yao, Analyst, 2012, 137, 2716–2722 RSC.
- Y. Kalmykova, K. Bjorklund, A. M. Stromvall and L. Blom, Water Res., 2013, 47, 1317–1328 CrossRef CAS PubMed.
- M. Smol, M. Włodarczyk-Makuła, K. Mielczarek, J. Bohdziewicz and D. Włóka, Polycyclic Aromat. Compd., 2016, 36, 20–39 CrossRef CAS.
- J. Długosz, Archives of Waste Management and Environmental protection, 2013, vol. 15, pp. 59–68 Search PubMed.
- Q. Y. Cai, C. H. Mo, Q. T. Wu, Q. Y. Zeng, A. Katsoyiannis and J. F. Ferard, J. Hazard. Mater., 2007, 142, 535–542 CrossRef CAS PubMed.
- P. Oleszczuk, S. E. Hale, J. Lehmann and G. Cornelissen, Bioresour. Technol., 2012, 111, 84–91 CrossRef CAS PubMed.
- C. V. Hung, B. D. Cam, P. T. N. Mai and B. Q. Dzung, Environ. Geochem. Health, 2014, 37, 133–146 CrossRef PubMed.
- G.-H. Wang, Y.-Q. Lei and H.-C. Song, Anal. Methods, 2014, 6, 7842–7847 RSC.
- J. Meng, C. Shi, B. Wei, W. Yu, C. Deng and X. Zhang, J. Chromatogr. A, 2011, 1218, 2841–2847 CrossRef CAS PubMed.
- Y. B. Luo, Q. W. Yu, B. F. Yuan and Y. Q. Feng, Talanta, 2012, 90, 123–131 CrossRef CAS PubMed.
- Q. Wu, M. Liu, X. Ma, W. Wang, C. Wang, X. Zang and Z. Wang, Microchim. Acta, 2012, 177, 23–30 CrossRef CAS.
- H. Heidari and H. Razmi, Talanta, 2012, 99, 13–21 CrossRef CAS PubMed.
- S. Mahpishanian and H. Sereshti, J. Chromatogr. A, 2016, 1443, 43–53 CrossRef CAS PubMed.
- M. Wang, S. Cui, X. Yang and W. Bi, Talanta, 2015, 132, 922–928 CrossRef CAS PubMed.
- A. Mehdinia, N. Khodaee and A. Jabbari, Anal. Chim. Acta, 2015, 868, 1–9 CrossRef CAS PubMed.
- W. A. W. Ibrahim, H. R. Nodeh, H. Y. Aboul-Enein and M. M. Sanagi, Crit. Rev. Anal. Chem., 2015, 45, 270–287 CrossRef PubMed.
- Q. Liu, J. Shi, T. Wang, F. Guo, L. Liu and G. Jiang, J. Chromatogr. A, 2012, 1257, 1–8 CrossRef CAS PubMed.
- Q. Liu, J. Shi, M. Cheng, G. Li, D. Cao and G. Jiang, Chem. Commun., 2012, 48, 1874 RSC.
- K. Petcharoen and A. Sirivat, Mater. Sci. Eng., B, 2012, 177, 421–427 CrossRef CAS.
- P. Yuan, M. Fan, D. Yang, H. He, D. Liu, A. Yuan, J. Zhu and T. Chen, J. Hazard. Mater., 2009, 166, 821–829 CrossRef CAS PubMed.
- J. Peng, Q. Liu, Z. Xu and J. Masliyah, Energy Fuels, 2012, 26, 2705–2710 CrossRef CAS.
- S. Li, N. Li, S. Yang, F. Liu and J. Zhou, J. Mater. Chem. A, 2014, 2, 94 CAS.
- L. Carlos, F. S. G. Einschlag, M. C. González and D. O. Mártire, Waste Water: Treat. Technol. Recent Anal. Dev., 2013, 63–78 CAS.
- J. Liu, Z. Zhao and G. Jiang, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS PubMed.
- W. L. Siew, T. S. Tang, Y. A. Tan and M. P. O. R. I. of Malaysia, PORIM: Test Methods, Palm Oil Research Institute of Malaysia, 1995 Search PubMed.
- W. W. Christie, Gas chromatography and lipids, Oily Press, Ayr, 1989, vol. 39 Search PubMed.
- K. Can, M. Ozmen and M. Ersoz, Colloids Surf., B, 2009, 71, 154–159 CrossRef CAS PubMed.
- B. Feng, R. Y. Hong, L. S. Wang, L. Guo, H. Z. Li, J. Ding, Y. Zheng and D. G. Wei, Colloids Surf., A, 2008, 328, 52–59 CrossRef CAS.
- E. Tahmasebi and Y. Yamini, Anal. Chim. Acta, 2012, 756, 13–22 CrossRef CAS PubMed.
- R. A. Perez, B. Albero and L. Tadeo, Anal. Methods, 2014, 6, 1941–1950 RSC.
- S. Shahabuddin, N. M. Sarih, S. Mohamad and J. J. Ching, Polymers, 2016, 8, 27 CrossRef.
- S. Shahabuddin, N. M. Sarih, S. Mohamad and S. N. A. Baharin, RSC Adv., 2016, 6, 43388–43400 RSC.
- S. Shahabuddin, N. M. Sarih, F. H. Ismail, M. M. Shahid and N. M. Huang, RSC Adv., 2015, 5, 83857–83867 RSC.
- Y. Liu, H. Li and J. Lin, Talanta, 2009, 77, 1037–1042 CrossRef CAS PubMed.
- S.-H. Huo and X.-P. Yan, Analyst, 2012, 137, 3445 RSC.
- J. Ding, Q. Gao, D. Luo, Z. Shi and Y. Feng, J. Chromatogr. A, 2010, 1217, 7351–7358 CrossRef CAS PubMed.
- Q. Han, Z. Wang, J. Xia, S. Chen, X. Zhang and M. Ding, Talanta, 2012, 101, 388–395 CrossRef CAS PubMed.
- A. Sarafraz-Yazdi, T. Rokhian, A. Amiri and F. Ghaemi, New J. Chem., 2015, 39, 5621–5627 RSC.
- S. Bakhshaei, M. A. Kamboh, H. R. Nodeh, S. Zain, S. Khalijah, M. Rozi, S. Mohamad and I. A. M. Mohialdeen, RSC Adv., 2016, 6, 77047–77058 RSC.
| This journal is © The Royal Society of Chemistry 2016 |