High performance solution-processed infrared photodiode based on ternary PbSxSe1−x colloidal quantum dots
Abstract
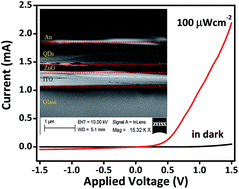
Semiconductor quantum dots (QDs) have been the subject for wide research studies owing to their quantum confinement effect. Photodetectors or photodiodes are recognized potential applications for QDs due to their high photosensitivity, solution processability and low cost of production. In this paper, a solution-processed near-infrared photodiode ITO/ZnO/PbSxSe1−x/Au, in which ternary PbSxSe1−x QDs act as the active layer and the ZnO interlayer acts as electron-transporting layer, was demonstrated. The photosensitive spectrum can be broadened by adjusting the molar fraction of ternary PbSxSe1−x QDs. The narrow band edge of absorption and photoluminescence exciton energy of PbSxSe1−x alloyed NCs were blue-shifted from the band edge of the same size PbSe QDs to the band edge of PbS QDs by controlling the S/(Se + S) molar ratio in the synthetic mixture. Efficient electron extraction was carried out by inserting a solution-processed ZnO interlayer between the indium-tin oxide (ITO) electrode and the active layer. Our experimental results show that the solution processing of the ZnO layer can lead to high-performance photodiodes by using photosensitized PbS0.4Se0.6 alloyed nanocrystals as the active layer. The effect of the thickness of the active layer on the device performance was briefly described and a maximum photoresponsivity and specific detectivity of 25.8 A/W and 1.30 × 1013 Jones, respectively, were obtained at a certain thickness under 100 μW cm−2 980 nm laser illumination. The devices are made stably by layer-by-layer ligand exchange treatment.

 Please wait while we load your content...
Please wait while we load your content...