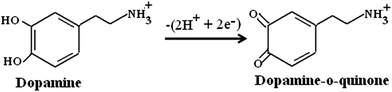Facile biosurfactant assisted biocompatible α-Fe2O3 nanorods and nanospheres synthesis, magneto physicochemical characteristics and their enhanced biomolecules sensing ability†
Sasikala Sundara,
Ramalakshmi Mariappanb,
Kim Minb and
Shakkthivel Piraman*ab
aSustainable Energy and Smart Materials Research Lab, Department of Nanoscience and Technology, Alagappa University, Karaikudi-630 002, Tamilnadu, India. E-mail: apsakthivel@yahoo.com; Fax: +91-4565-225202; Tel: +91-4565-223-372
bCollege of Science and Technology, Dongguk University, Gyeongju-780 714, South Korea. Tel: +82-54-740-4984
First published on 28th July 2016
Abstract
Recently, non-spherical magnetic iron oxide nanoparticles have attracted much attention, because of their shape-dependant potential applications in biosensors and magnetic storage devices. In this work, a simple, novel, environmentally friendly, energy inexpensive, toxic free synthetic approach was utilized to synthesize α-Fe2O3 nanorods with an aspect ratio of 10 and homogeneously dispersed 30 nm α-Fe2O3 nanospheres (from TEM and FE-SEM images) using non-toxic “Centellasaponin” biosurfactant/capping agent tapping the big polar and non-polar head saponin induced anisotropic growth. The α-Fe2O3 nanorods and nanospheres synthesized by the template-free, low cost, simple, energy intensive route were confirmed by FTIR, XRD and the X-ray photoelectron spectroscopy by the appearance of electron binding energy at 710.74 eV and 724.2 eV corresponding to 2P3/2 and 2P1/2 core levels of α-Fe2O3 nanoparticles. Nano α-Fe2O3 spheres and rods synthesized at different temperatures and various concentrations exhibit superparamagnetic characteristics consisting of a single magnetic domain of nanoparticles with no discernible coercivity and remanance with not ending magnetic saturation hysteresis and will find potential applications in magnetic recording and targeted drug delivery. Nano α-Fe2O3 magnetic particles displayed excellent electrochemical sensing activities towards dopamine and uric acid with 320 mV and 260 mV wider separation on α-Fe2O3 nanorods and α-Fe2O3 nanospheres, respectively at 90–170 mV reduced potential with 100% increased current response. With good selectivity and sensitivity, the α-Fe2O3 nanostructures could be applied in the determination of dopamine in injectable medicine and uric acid in urine samples.
1. Introduction
The morphologically-controlled synthesis of inorganic nanocrystalline materials has drawn considerable interest in recent years, because the size and shape of these materials play a key role in determining their physico-chemical properties compared with those of the corresponding bulk counterpart.1,2 Among the inorganic materials, polymorphs of iron oxides, such as hematite, maghemite, and magnetite have received a great deal of attention due to their numerous fundamental and technological applications.3–9 Hematite (α-Fe2O3) has attracted the significant attention of researchers, because, it is non-toxic, economical, environmentally friendly, highly stable under ambient conditions, and has multiple functions. Particularly, one-dimensional (1D) α-Fe2O3 nanorods have been extensively investigated for applications in sensors,10–12 lithium batteries,13,14 catalysts,15 pigments,16 and magnetic devices,17 because their magnetic properties are greatly dependent on the nanorod size and shape.18–21Stimulated by both the promising applications and the fascinating chemical and physical properties such as electrical and thermal conductivity, structural characteristics, and magnetic properties of nanoscale iron oxide, considerable efforts have been devoted to the synthesis of α-Fe2O3 nanostructure materials with different morphologies. Up to now, various morphologies of α-Fe2O3, such as nanoparticles,22 nanowires,8 nanorods,23 nanotubes,24 hollow spheres,25 nanobelts,26 nanoplates,27 and complex hierarchical structures,28–31 have been prepared through various methods like hydrothermal methods, sol–gel processes, chemical precipitation, electrochemical and template methods. The forced hydrolysis of a homogeneous solution containing ferric ions method has been successfully used to synthesize hematite nanoparticles.32 Nevertheless, the interactions between the ferric/ferrous ions and the small organic molecules or complicated inorganic compounds have also been proved to play an important role in the formation of morphologically controlled iron oxide nanomaterials.33,34 For example, hematite nanorings and nanotubes have been synthesized hydrothermally in the presence of ammonium dihydrogen phosphate.35 However, the synthesis of hematite nanostructures with controlled shape and size still needs to be improved further, especially through controlling/adjusting the interactions between the reagents via a template-free synthesis strategy. In our previous report, we have deliberated the different polymorphs of iron oxide synthesis using greener saponin,36 and also γ-Fe2O3 nanostructures were utilized for the biomolecule sensing.37 To the best of our knowledge, no reports on the synthesis of α-Fe2O3 nanorods using a natural extract have been published to date.
Here in, we have developed a fast, clean, efficient, low-cost and eco-friendly single step green chemistry approach for the synthesis of biocompatible hematite iron oxide nanostructures (nanorods and nanospheres) by the co-precipitation of different oxidation states of iron ions using a Centellasaponin (CS) rich biosurfactant (Centella asiatica (Vallarai-Tamil name) leaf extract). Additionally, the present study relates to the electrocatalytic activity of the synthesized α-Fe2O3 nanostructures on a glassy carbon electrode (GCE) to develop a novel sensor for the selective and sensitive detection of dopamine (DA) and uric acid (UA) individually and simultaneously. Compared with the bare GCE, the peak potentials for these species on α-Fe2O3 nanorods and nanospheres modified GCE shifted negatively with enhanced current response, which was ascribed to the good electrocatalytic activity of the α-Fe2O3 nanostructures.
2. Experimental section
2.1 Synthesis of α-Fe2O3 nanorods and nanospheres
Reagent grade iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) sulphate heptahydrate (FeSO4·7H2O), sodium hydroxide (NaOH), uric acid (UA) and dopamine (DA) were purchased from E-Merck Specialities Products, and were used without further purification. Centella asiatica leaves were purchased from the local market, in Karaikudi, India to prepare the extract.The α-Fe2O3 nanostructures were prepared by an aqueous co-precipitation method using FeCl3·6H2O and FeSO4·7H2O. In a typical experimental procedure, 30 ml of 0.1 M FeCl3·6H2O solution was mixed with 15 ml of 0.1 M FeSO4·7H2O solution in a 3-necked round bottom flask. After 30 minutes, the saponin rich biosurfactant Centellasaponin (CS) was added to the above mixture and allowed to react for 1 h. After the reaction time of 1 h, NaOH solution was added slowly to the reaction mixture under vigorous stirring until pH 13 was reached. The mixture was stirred for another 2 h for completion of the reaction. The solution color changed slowly from orange to black, finally a reddish brown precipitate was formed. The synthesis process was performed at room temperature under an ambient atmosphere without the protection of inert gases or a vacuum atmosphere. The supernatant solution was decanted and the resulting solid powder products were washed with double distilled water and dried at room temperature. The effect of the biosurfactant concentration on the co-precipitation of Fe3+ and Fe2+ ions and the reaction progress for nanospheres and nanorods formation at different reaction times (10–180 min) were investigated. The influence of reaction temperature on the structural and textural formation of the iron oxide nanostructures was also investigated at the reaction temperatures of 30, 40 and 60 °C.
2.2 Characterization of iron oxide nanostructures
The characteristics of the α-Fe2O3 nanostructures were studied by means of field emission scanning electron microscopy (FE-SEM) using an SEM Hitachi MODEL S-4800 instrument. A JEOL TEM 2010 transmission electron microscope (TEM) operating at 200 kV was used to obtain TEM images. An X-ray diffraction study (XPERT-PRO with Cu Kα radiation (λ = 0.154060 nm), PANlytical X’Pert Pro Diffractometer) was used to study the structural phase identification of the synthesized iron oxide samples. A Fourier transform infrared spectrometric study (FTIR, Nicolet 5700) was performed to analyze the surface functional characteristics and the chemical composition of the synthesized iron oxide nanoparticles. In order to determine the oxidation states of Fe in the synthesized α-Fe2O3 samples, X-ray photoemission spectra (XPS) were recorded using a Kratos ASIS-HS X-ray photoelectron spectroscope equipped with a standard and monochromatic source (Al Kα) and operated at 150 W (15 kV, 10 mA). The magnetization curves and hysteresis loop of the α-Fe2O3 nanostructures (rods and spheres) were characterized with a Lake Shore model 7300 Vibrating sample magnetometer (VSM).2.3 Fabrication of iron oxide nanostructures modified GCE
The surface of the GCE was polished to a mirror-like surface finish using 1, 0.3 and 0.05 μm alumina slurry, and successively washed with distilled water in an ultrasonic bath, electrochemically cleaned in 0.1 M HCl and dried in air. Then, 1 mg of α-Fe2O3 iron oxide nanoparticles (nanorods/nanospheres) were dispersed in 3 ml of ethanol under ultrasonication and 5 μl of the suspension was dropped/casted on the surface of the electrochemically cleaned GCE and dried naturally in air at room temperature. The α-Fe2O3 modified GCE was activated in 7.4 M phosphate buffer solution (PBS) by successive cyclic scans between −0.2 and +0.8 V. Before and after each experiment, the modified GCE was washed with distilled water and reactivated in PBS as explained above.2.4 Electrocatalytic oxidation of UA and DA on iron oxide modified GCE
The electrocatalytic oxidation of DA and UA was performed using the synthesized iron oxide nanoparticles modified GCE and on a bare GCE in 0.5 mM DA and UA solutions. Nitrogen gas was purged in the freshly prepared DA and UA solutions for 5 min to eliminate dissolved oxygen from the solutions and was flowed over the solution to avoid interference from atmospheric oxygen in the electrochemical oxidation of the analyte. Cyclic voltammetric (CV) tests were performed in the voltage range of −0.2 − +0.8 V at a scan rate of 50 mV s−1 using a CHI6131D Electrochemical Impedance Analyzer (USA) with the as-modified electrode and a bare GCE as the working electrode, a platinum wire and saturated calomel electrodes were used as the counter and reference electrode, respectively.3. Results and discussion
To extensively scrutinize the morphology of the synthesized α-Fe2O3 nanostructures, we carried out FE-SEM and TEM experiments on iron oxide nanoparticles synthesized using various concentrations (1%, 2% and 10%) of CS. From the FE-SEM and TEM (Fig. 1d and 2a) measurements, the individual nanorods of α-Fe2O3 synthesized with 2% CS were found to have an average length and diameter of 2700 nm and 340 nm, respectively, with an aspect ratio of 8. However, the aspect ratio of the α-Fe2O3 samples synthesized with 2% CS was not too high, as α-Fe2O3 can form bigger nanorods. This may be due to the agglomeration of the particulates in both the longitudinal and transverse directions during the synthesis. However, the longitudinal growth was faster than the lateral one. In addition, significantly, there are no side branches and cross linking between the nanorods, which is a major advantage for the controlled longitudinal growth process of α-Fe2O3 and the use of single nanorods in some specialized applications. Here, the saponin molecule present in the CS is responsible for the formation of nanorods and allows the growth mainly along the [104] and [110] plane as seen from the XRD spectral lines (Fig. 1b and e). The aspect ratio of the nanorods formed in 1% CS was around 10 (Fig. 1a) which is a little bit higher than those grown in 2% CS, this is due to the much reduced diameters of the nanorods relative to the length of the α-Fe2O3 nanorods synthesized in 1% CS (1350 nm and 133 nm). The reduction in length of the nanorod suggests that the nanoparticles are preferably agglomerated along the transverse direction leading to the formation of smaller nanorods.35,36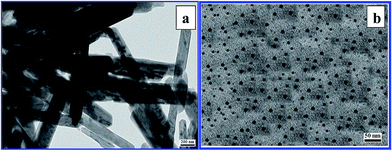 | ||
| Fig. 2 TEM images of α-Fe2O3 nanorods and nanospheres synthesized at 30 °C in 3 h with 2% (a) and 10% (b) CS. | ||
Furthermore, with 10% concentration of CS, only α-Fe2O3 nanospherical particles were formed (Fig. 1g and 2b) which are homogeneously dispersed and highly crystalline, and the size of the formed α-Fe2O3 spherical nanoparticles was around 10 nm. This is because, the surfactant may form micelles on the surface of the particles after nucleation, thereby prohibiting further growth and keeping the α-Fe2O3 particles as nanospheres. The ability of the micelles to inhibit the growth of the particles through a particular facet becomes stronger in response to an increase in the surfactant concentration, which initially reduces the average particle size, and finally leads to the formation of homogeneously dispersed spherical nanoparticles. Moreover, higher surfactant molecules can provide more opportunities for contact with the surface of the nucleus of the spherical α-Fe2O3 nanoparticles in any direction. This also means that the distribution of the surfactant in all the directions on the surface of the α-Fe2O3 nanoparticles is highly isotropic. It was found that a better size distribution in the process of the crystal growth could be obtained with the more befitting amount of the surfactant used in the experiment. From the above observations, it is clear that the use of CS has the ability to change the morphology of nanostructures through the chemical structure of CS responsible for micelle formation. The chemical structure of different types of CS present in Centella asiatica leaf extract is presented in Fig. S1.†
To investigate the influence of reaction temperature on the evolution process of α-Fe2O3 nanorods and nanospheres in 2% CS with a reaction time of 3 h, the reaction was carried out at 30 °C, 40 °C and 60 °C (Fig. 4). Furthermore, the growth process of the α-Fe2O3 nanorods was examined at various reaction times of 10 min, 30 min and 180 min in 2% CS at 30 °C and their FE-SEM micrographs are presented in Fig. S2.† When the reaction period was 10 min, homogeneously dispersed uniform sized nanospheres were formed, however, with a prolonged reaction time of 3 h well dispersed nanorods without any side branches and cross linking were observed, but with a shorter reaction time of 10 min only spherical α-Fe2O3 nanoparticles which were 20 nm in size were observed and a reaction time of 30 min resulted in aggregated spherical nanoparticles which were 45 nm in size. Hematite nanorods were gradually formed through the aggregation of nanospheres, which may be related to both the intrinsic crystal nature and the selective adsorption of CS on the different faces of the α-Fe2O3 nanocrystals. A lower concentration of CS (1% and 2%) did not affect the structural morphology of the α-Fe2O3 nanorods, whereas when 10% of CS was added to the reaction system, 10 nm monodispersed nanospherical particles were obtained. Hence, it has been confirmed that the CS biosurfactant plays a major role in the growth and formation of α-Fe2O3 nanorods and nanospheres.37
A possible mechanism for the formation of α-Fe2O3 nanorods has been proposed and is presented in Fig. 3. The whole process for the formation of α-Fe2O3 nanorods comprises nucleation, surface regularity, growth and oriented attachment. Initially, the crystals nucleate from the precursor solution, when the crystal is nucleated from the solution, the growth habit emerges simultaneously. The freshly formed nanocrystals are unstable due to their high surface energy and they tend to aggregate rapidly, probably driven by the oriented attachment process. In our system, the nucleation and growth rates are very fast due to the presence of saponin molecules which are present in CS (Fig. S1†). Also, the –OH group of saponin present in CS can attach easily to the surface of the crystal faces of iron oxide which affects the crystallographic orientation. The nanorods formed are bound by 104 and 110 planes as the result of the kinetically controlled process. Under the capping effect of CS, α-Fe2O3 crystals preferentially grow along the long chain of CS as rod-like crystals via oriented attachment, where the strong van der Waals attraction between the spherical nanoparticles results in progressive aggregation of the nanoparticles.38,39 However, in the case of the higher concentration of CS (10%), α-Fe2O3 nanospherical formation was observed, which was instigated by micelle formation of the surfactant.35 Here, the CS biosurfactant strongly competes with the iron oxide nanostructures and helps to retain the nanospherical structure, in which only homogeneous nanospherical particles are formed. The biosurfactant molecules give rise to repulsive forces between the nucleated particles, thereby prohibiting further growth in such a way that the nanospheres are kept apart in an homogeneously dispersed state. The desired shape of the iron oxide (α-Fe2O3) nanostructures could be tailored by designing the experimental parameters to exploit the natural biosurfactant (CS) to achieve the fascinating properties of structural and morphological dependent characteristics.
The temperature dependent formation of α-Fe2O3 nanoparticles can be visualized in a series of FE-SEM images presented in Fig. 4. The star like morphology and agglomerated hematite spherical particles were generated via the hydrolysis of ferric and ferrous ions in the presence of CS at elevated temperatures. At 30 °C with a 3 h nanoparticle formation time, well defined bigger α-Fe2O3 nanorods with an aspect ratio of 10 were formed. On the other hand, at 40 °C, no nanorods formation was observed. Here, the temperature influenced the ionic transport of the precursor iron ions leading to the formation of dismantled glass like embryos with a star like morphology and a particle size of about 30 ± 3 nm (Fig. 4c) instead, the surface was mainly covered with seemingly well crystallized spherical particles (nanostructures). When the reaction temperature was further increased to 60 °C, bigger hematite spherical nanoparticles were formed (55 ±5 nm) accompanied by the agglomeration of the α-Fe2O3 spherical nanoparticles in the 2% CS (Fig. 4e).
The XRD patterns of the iron oxide nanoparticles synthesized with different concentrations of CS biosurfactant at ambient and at different reaction temperatures are illustrated in Fig. 1 and 4. All the diffraction peaks in the XRD pattern can be indexed to the hematite (α-Fe2O3) crystal structure (JCPDS card-89-8104) indicating pure and highly crystalline products. The hematite crystals have a rhombohedrally centered hexagonal structure of the corundum type with a close-packed lattice in which two thirds of the octahedral sites are occupied by Fe3p ions. In a typical crystal unit, each Fe atom is surrounded by six O atoms, whereas each O atom is bound to four Fe atoms. The X-ray diffraction technique can yield a great deal of structural information, and phase identification of the synthesized α-Fe2O3 nanorods and α-Fe2O3 nanospheres.40 A rhombohedral crystal structure was observed for the nanorods synthesized with 1% and 2% CS and for the nanospheres synthesized with 10% CS. Without any differences in the XRD peak pattern, the α-Fe2O3 nanospheres exhibited higher intensity peaks compared to the α-Fe2O3 nanorods. This was also inferred from the increased number of spheroid shaped particles observed in the FE-SEM and TEM images for the α-Fe2O3 nanoparticles synthesized with 10% CS (Fig. 1g and 2b). Further, in the XRD patterns of the α-Fe2O3 nanorods synthesized in 1% and 2% CS, higher intensity peaks were observed at 2θ values of 35.5° and 33.4° for the 110 and 104 planes, respectively, indicating that the α-Fe2O3 nanorods grow along the 110 and 104 directions (Fig. 1b and e).
However, when the reaction temperature was raised from 30 °C to 40 °C, star shaped nano-uniformed particles were formed and by increasing the reaction temperature to 60 °C, agglomerated α-Fe2O3 nanospheres were formed (Fig. 4c and e). When the reaction temperature was increased, the full-width at half-maximum (FWHM) of the reflection peaks decreased and the X-ray reflection peaks became sharper. It can be seen that the XRD patterns of the Fe2O3 samples synthesized at 30 °C, 40 °C and 60 °C conform with the rhombohedral structure of the α-Fe2O3 phase with a = 5.038 Å and c = 13.772 Å values. The XRD data compared well with the reported data (JCPDS 89-8104), it was found that the main crystalline phase of the synthesized iron oxide was α-Fe2O3 (Fig. 4b, d and f). This implies that temperature does not influence the ultimate production of α-Fe2O3, but has an influence on the oriented attachment process. From the Debye–Scherrer equation, the average crystallite size of the synthesized α-Fe2O3 products at 40 °C and 60 °C were calculated to be 43 nm and 62 nm, respectively, the particle size increased when the reaction temperature was increased and this was corroborated by the particle sizes observed in the FE-SEM images. Bigger particles were obtained at higher temperatures, because the molecules on the surface of a particle are energetically less stable than those that are already well ordered and packed in the interior. Large particles with their lower surface to volume ratio result in a lower energy state. The molecules on the surface of a small particle will tend to dissolve and diffuse through a solution with the temperature effect. As the system tries to lower its overall energy, precipitates of nanocrystals tend to agglomerate to bigger particles. Therefore, the bigger particles grow larger and larger at the expense of the neighboring smaller particles. At the same time, the diffusion process can be accelerated by an increase in reaction temperature, promoting the growth of crystal grains more effectively.41 The dependence of the particle size on the temperature suggests that the reaction temperature plays an important role in tailoring the morphology of the nanoparticles.
Presently, XRD is not sufficient to qualitatively determine whether the cation vacancies in α-Fe2O3 are partially or fully ordered over the octahedral sites.42 Moreover, the obtained XRD patterns cannot provide enough evidence to confirm that the sample is α-Fe2O3. At first, we noticed that the colors of the samples were the characteristic color of α-Fe2O3 (reddish brown) which is significantly different from that of the other phase of iron oxide like Fe3O4 (black). The chemical composition of Fe2O3 as opposed to Fe3O4 was further confirmed by XPS analysis of the Fe2p orbitals. Thus additionally, XPS analysis was performed to confirm the valence states of iron ions in the α-Fe2O3 nanorods and nanospheres synthesized with different concentration of CS and the resulting core-level XPS spectra of the nanorods and nanospheres in the Fe2p region are presented in Fig. 1c, f and i. The centers of the electron binding energy of the two major peaks were observed at 710.74 and 724.2 eV for the nanorods synthesized with 1% CS, 710.87 and 724.4 eV for the nanorods synthesized with 2% CS, and 710.65 and 724.8 eV for the nanospheres synthesized with 10% CS corresponding to the 2P3/2 and 2P1/2 core levels of α-Fe2O3 nanoparticles. Small shake-up satellite signals (shoulder peak) appeared at around 719.2 and 719.3 eV (nanorods) and 719.7 eV (nanospheres) which are also indicators of α-Fe2O3 nanoparticles. The shake-up satellite structures at the higher binding energy sides of the main peaks are the fingerprints of the electronic structure of Fe3+ and clearly indicate that Fe2+ is absent. According to the XPS spectra, the positions of the peaks as well as the shape of the 2P spectra agree well with those reported earlier for the Fe3+ state.43,44 The XPS patterns were in good agreement with the XRD data and confirmed that the pure phase of α-Fe2O3 could be formed by use of this facile method.
The chemical structure of the synthesized α-Fe2O3 was verified by carrying out FTIR studies, the FTIR spectra of the different morphological structures of the α-Fe2O3 nanorods and nanospheres synthesized with different concentrations of CS and at various reaction temperatures are presented in Fig. S3 and S4†, respectively. The appearance of bands at 567 and 445 cm−1 in all the spectra of the synthesized samples at various conditions correlated with the Fe–O stretching vibrational mode, which confirms the presence of the α-Fe2O3 phase.45 The formation of Fe–O in the presence of only Fe3+ in the iron oxide structure was confirmed by the FTIR results and was also corroborated by the XRD and XPS analysis results.
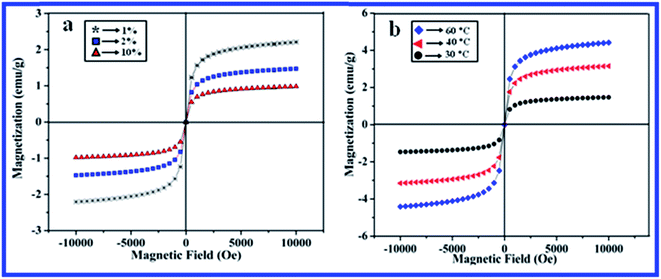
The magnetic properties of materials have been proved to be highly dependent on many factors including the size and shape of the materials.46 Bulk hematite is weakly ferromagnetic at 298 K with a Neel temperature of 955 K and undergoes a “spin-flop” (Morin) transition at 263 K, in which the magnetic moments change their orientation.47 However, the α-Fe2O3 nanoparticles can exhibit anti-ferromagnetic, ferromagnetic and superparamagnetic behaviors, depending strongly on the size, shape and porosity favored by the preparation conditions of the materials. Therefore, it is of great interest to investigate the influence of the nanostructure on the magnetic properties. Magnetization measurements of the synthesized α-Fe2O3 nanostructures with different morphologies were carried out in an applied magnetic field sweeping from −10 to 10 kOe. Fig. 5a and b depicts the room temperature hysteresis curves of the α-Fe2O3 nanorods and nanospheres synthesized with different concentrations of the saponin rich biosurfactant (1–10%) and at different reaction temperatures (30–60 °C). It can be seen that in all the samples, coercivity and remanance values are not discernible at 300 K, indicating the existence of superparamagnetic behavior with a non-ending saturation magnetization similar to that reported in the literature.48 Superparamagnetism occurs in nanoparticles consisting of a single magnetic domain with a diameter between 3 and 50 nm.49 The reason for the superparamagnetism exhibited by the synthesized α-Fe2O3 nanoparticles is that their definite size results in each particle exhibiting a single magnetic domain character and the energy barrier for its spin reversal is easily overcome by thermal vibrations.
Magnetization (Ms) at the maximum applied magnetic field of 10 kOe for the samples prepared with 1%, 2% and 10% CS concentration at 30 °C was 2.17, 1.45 and 0.92 emu g−1, respectively (Fig. 5a). In addition, when the concentration of CS was 10%, the average diameter of the particles was about 10 nm and the presence of organic moieties on the α-Fe2O3 nanoparticles was the main reason for the reduction of Ms in this work. Further, the high percentage of surface atoms was linked to the surfactant (CS). The surface effects generate a magnetic “dead layer” on the nanoparticles surfaces, which is commonly observed in small magnetic nanoparticles systems.50 However, the value of Ms was improved with an increase in reaction temperature at 30 °C (1.45 emu g−1), 40 °C (3.21 em g−1) and 60 °C (4.42 emu g−1) as shown in Fig. 5b. Moreover, the Ms value of all the samples was lower than that of the bulk α-Fe2O3 material, this can be ascribed to the non-collinear structure of the surfactant located on the surface of the particles.51,52
The electrocatalytic oxidation of DA and UA on the α-Fe2O3 nanorods and nanospheres modified GCE in 7.4 PBS was carried out in the potential range of −0.2 − +0.8 V at a 50 mV s−1 scan rate by cyclic voltammetry. Fig. 6 and 7 show the voltammetric responses of the DA and UA at the bare GCE and α-Fe2O3 nanorods and nanospheres modified GCE. The α-Fe2O3 nanorods and nanospheres modified GCE reduced the anodic over potentials of DA and UA and well-defined anodic peaks for the analyte molecules were observed compared to those observed with the bare GCE. At the bare GCE, UA shows an oxidation peak at 0.52 V. It is well known that the oxidation of UA at a bare GCE is generally believed to be totally irreversible and requires a high over potential.36 However, the UA oxidation voltammogram obtained with the α-Fe2O3 nanorods and nanospheres modified GCE showed an oxidation wave at 0.35 V and 0.37 V potential, respectively ,with an increased current response (100% for α-Fe2O3 nanorods) (Fig. 7). For DA, an oxidation peak at 0.30 V at the bare GCE was observed. Conversely, in the DA voltammogram an oxidation wave at a reduced potential of 0.18 and 0.21 V was observed for the α-Fe2O3 nanorods and nanospheres modified GC electrodes, respectively (Fig. 6) with an enhanced peak current, which is attributed to the excellent absorption ability of the α-Fe2O3 nanorods and nanospheres towards the biomolecules.
 | ||
| Fig. 6 Cyclic voltammetric response of 0.5 × 10−3 M DA in 7.4 PBS at a scan rate of 50 mV s−1 on a bare GCE (a), α-Fe2O3 nanospheres (b) and α-Fe2O3 nanorods (c) modified GCE. | ||
 | ||
| Fig. 7 Cyclic voltammetric response of 0.5 × 10−3 M UA in 7.4 PBS at a scan rate of 50 mV s−1 on a bare GCE (a), α-Fe2O3 nanospheres (b) and α-Fe2O3 nanorods (c) modified GCE. | ||
FTIR studies showed that the Fe2O3 layer contains free surface –OH group which are negatively charged. These negatively charged particles attract positively charged DA at a physiological pH of 7.4, and form hydrogen bonds with the –NH2 group of DA. This essentially results in a hydroxyl bond which facilitates electron transfer through the –NH2 group of DA and the –OH (surface hydroxyl group) of Fe2O3. Also, the induced magnetization of the Fe2O3 nanoparticles domains on applying potential results in an increased current response when compared to that of the bare GCE. It is reported that the redox process of dopamine proceeds via a 2e−, 2H+ process.53 Based on the above discussion and the previous reports, the electrochemical redox mechanism of DA at the α-Fe2O3/GCE can be explained by Scheme 1.
Likewise, UA oxidation involves a two electron transfer and hence the number of protons taking part in the reaction is also suggested to be two as shown in Scheme 2, which represents the electrochemical oxidation mechanism for UA at the α-Fe2O3 nanoparticles/GCE.54 Also, a slight negative shift in potential was observed along with the enhancement in current which is attributed to the good electrical conductivity, the large surface area and the increased number of electroactive interaction sites in the α-Fe2O3 nanoparticles/GCE, all of which combine to offer increased mass transport and easier accessibility to the active sites.
Compared to the earlier literature,12,54–64 the obtained results confirm that the sensing ability of the facile synthesized and fabricated α-Fe2O3 based electrode is very good, and oxidizes the analyte at a much reduced potential and an increased current response, as shown in Table 1.
| Performance of uric acid sensors | Performance of dopamine sensors | ||||
|---|---|---|---|---|---|
| Electrode system | Sensing potential (V) | Reference | Electrode system | Sensing potential (V) | Reference |
| α-Fe2O3/GCE | 0.39 | 12 | GC/α-Fe2O3 | 0.23 | 59 |
| FeMWCNTs/MCPE | 0.57 | 54 | Au@Pd–RGO/GCE | 0.22 | 60 |
| AuMCs/SF-GR/GCE | 0.54 | 55 | HNP–PtTi alloy/GCE | 0.41 | 61 |
| PG/GCE | 0.38 | 56 | Mo–rGO/PI film/GCE | 0.23 | 62 |
| GOU/GCE | 0.47 | 57 | N–G/NiTsPc/GCE | 0.24 | 63 |
| AuNPs(EDAS)–rGO/GCE | 0.40 | 58 | GO–Ag/PLL/GCE | 0.23 | 64 |
| This work α-Fe2O3/GCE nanospheres | 0.37 | This work α-Fe2O3/GCE nanospheres | 0.21 | ||
| Nanorods | 0.35 | — | Nanorods | 0.18 | — |
The above results demonstrate that the α-Fe2O3 modified GCE not only accelerates the oxidation of DA and UA, but also dramatically enlarges the peak separation between the DA and UA molecules. The enlarged anodic peak potential separation coupled with the increased sensitivity renders the simultaneous determination of DA and UA feasible. Here, the α-Fe2O3 nanorods and nanospheres modified GCE exhibit two well-defined separate anodic peaks for the simultaneous oxidation of DA and UA with an enhanced current response (Fig. 8). The presence of functional groups on the α-Fe2O3 modified GCE resolved the mixed voltammetric response of these species (DA and UA) into two well-defined voltammetric peaks at potentials of 190 mV and 510 mV (320 mV peak separation) for the α-Fe2O3 nanorods and 220 mV and 480 mV (260 mV peak separation) for the α-Fe2O3 nanospheres modified GCE, respectively. Further the peak separation between DA and UA on α-Fe2O3 nanorods and α-Fe2O3 nanospheres is sufficient to oxidize them simultaneously as two well-defined separate peaks for easy determination, the α-Fe2O3 nanorods modified GCE is the best as it exhibits 320 mV wide separations between the DA and UA voltammetric response with an increased peak current.
 | ||
| Fig. 8 Simultaneous determination of DA and UA on bare (a), α-Fe2O3 nanospheres (b) and α-Fe2O3 nanorods (c) modified GCE at 50 mV s−1 in 7.4 PBS solution containing 0.5 × 10−3 M (DA and UA). | ||
The voltammetric responses indicate that the electrocatalytic reaction on the α-Fe2O3/GCE facilitates electron transfer between the electrode and the analyte molecules, as a result, the electrochemical oxidation of DA and UA becomes easier. The α-Fe2O3 can act as a promoter to increase the rate of electron transfer, lowering the over potential of DA and UA on the modified electrodes, it is clear that the synthesized α-Fe2O3 nanostructures modified GC electrodes can be successfully used for the determination of biomolecules and bioelectronics/biosensors and also for biomagnetic applications. The facile, environmentally friendly and toxic free synthesis method described herein, could be applied in the design of morphology and definite size dependent magnetic and electrocatalytic phase controlled iron oxide characteristics.
4. Conclusions
In summary, a facile method has been developed for the synthesis of superparamagnetic nano α-Fe2O3 hematite nanorods (2700 nm length and 340 nm dia) and nanospheres (10–30 nm) through saponin rich biosurfactant assisted co-precipitation, which is simple, cost effective and environmentally friendly. Magnetic hysteresis measurements show that the size and shape of the hematite nanostructures have important effects on their magnetic properties. The hematite nanostructures were applied in the modification of a GCE, and exhibited high electrocatalytic activities towards the oxidation of DA and UA by significantly decreasing their oxidation over potentials (90–170 mV) and enhancing their peak currents. A large peak separation (260–320 mV) between DA and UA was observed using cyclic voltammetry indicating that the α-Fe2O3 nanostructures facilitated their simultaneous determination. And promisingly, the high surface area and 1D nanostructure of α-Fe2O3 could be readily extended to the detection of other clinically important antigens to develop other simple and practical biosensors. Therefore, we strongly believe that the use of a saponin rich biosurfactant enables the large scale production of biocompatible iron oxide nanostructures that can be used as vehicles for biomedical/sensing applications.References
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- C. Wu, P. Yin, X. Zhu, C. OuYang and Y. Xie, J. Phys. Chem. B, 2006, 110, 17806–17812 CrossRef CAS PubMed.
- L.-Y. Hung, J.-C. Chang, Y. C. Tsai, C.-C. Huang, C.-P. Chang, C.-S. Yeh and G.-B. Lee, Nanomedicine, 2014, 10, 819–829 CAS.
- Z. Dong, P. Kashkarov and H. Zhang, Nanoscale, 2010, 2, 524–528 RSC.
- L. Suber, P. Imperatori, A. Mari, G. Marchegiani, M. Vasquez Mansilla, D. Fiorani, W. R. Plunkett, D. Rinaldi, C. Cannas, G. Ennas and D. Peddis, Phys. Chem. Chem. Phys., 2010, 12, 6984–6989 RSC.
- X. Jia, F. Yue, X. Chen, H.-B. Pan, W.-G. Liu and J.-Y. Liu, RSC Adv., 2014, 4, 42899–42904 RSC.
- J. Chen, L. N. Xu and W. Y. Li, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- R. Suresh, R. Prabu, A. Vijayaraj, K. Giribabu, A. Stephen and V. Narayanan, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2012, 42, 303–307 CrossRef CAS.
- B. Sun, J. Horvat, H. S. Kim, W. S. Kim, J. Ahn and G. X. Wang, J. Phys. Chem. C, 2010, 114, 18753–18761 CAS.
- Z. Xiao, Y. Xia, Z. Ren, Z. Liu, G. Xu, C. Chao, X. Li, G. Shen and G. Han, J. Mater. Chem., 2012, 22, 20566–20573 RSC.
- T. Cheng, Z. Y. Fang, Q. X. Hu, K. D. Han, X. Z. Yang and Y. J. Zhang, Catal. Commun., 2007, 8, 1167–1171 CrossRef CAS.
- F. Bondioli, A. M. Ferrari, C. Leonelli and T. Manfredini, Mater. Res. Bull., 1998, 33, 723–729 CrossRef CAS.
- S. Mitra, S. Das, K. Mandal and S. Chaudhuri, Nanotechnology, 2007, 18, 275608–275609 CrossRef.
- Z. Jing and S. Wu, Mater. Lett., 2004, 58, 3637–3640 CrossRef CAS.
- L. Liu, H. Z. Kou, W. Mo, H. Liu and Y. Wang, J. Phys. Chem. B, 2006, 110, 15218–15223 CrossRef CAS PubMed.
- S. Zeng, K. Tang and T. Li, J. Colloid Interface Sci., 2007, 312, 513–521 CrossRef CAS PubMed.
- Y. Zhao, C. W. Dunhill, Y. Zhu, D. H. Gregory, Y. L. Kockenberger, W. Hu, I. Ahmad and D. G. McCartney, Chem. Mater., 2007, 19, 916–921 CrossRef CAS.
- K. Woo, J. W. Hong, S. M. Choi, H. W. Lee, J. P. Ahn, C. S. Kim and S. W. Lee, Chem. Mater., 2004, 16, 2814–2818 CrossRef CAS.
- S. F. Si, C. H. Li, X. Wang, Q. Peng and T. D. Li, Sens. Actuators, B, 2006, 119, 52–56 CrossRef CAS.
- C. J. Jia, L. D. Sun, Z. G. Yan, L. P. You, F. Luo, X. D. Han, Y. C. Pang, Z. Zhang and C. H. Yan, Angew. Chem., Int. Ed., 2005, 44, 4328–4333 CrossRef CAS PubMed.
- Z. C. Wu, K. Yu, S. D. Zhang and Y. Xie, J. Phys. Chem. C, 2008, 112, 11307–11313 CAS.
- X. G. Wen, S. H. Wang, Y. Ding, Z. L. Wang and S. H. Yang, J. Phys. Chem. B, 2005, 109, 215–220 CrossRef CAS PubMed.
- M. F. Casula, Y. W. Jun, D. J. Zaziski, E. M. Chan, A. Corrias and A. P. Alivisatos, J. Am. Chem. Soc., 2006, 128, 1675–1682 CrossRef CAS PubMed.
- K. He, C. Y. Xu, L. Zhen and W. Z. Shao, Mater. Lett., 2008, 62, 739–742 CrossRef CAS.
- L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- L. P. Zhu, H. M. Xiao and S. Y. Fu, Cryst. Growth Des., 2007, 7, 177–182 CAS.
- P. Das, B. Mondala and K. Mukherjee, RSC Adv., 2014, 4, 31879–31886 RSC.
- S. Hamada and E. Matijevic, J. Chem. Soc., Faraday Trans. 1, 1982, 78, 2147–2156 RSC.
- H. M. Fan, G. J. You, Y. Li, Z. Zheng, H. R. Tan, Z. X. Shen, S. H. Tang and Y. P. Feng, J. Phys. Chem. C, 2009, 113, 9928–9935 CAS.
- Z. Pu, M. Cao, J. Yang, K. Huang and C. Hu, Nanotechnology, 2006, 17, 799–804 CrossRef CAS.
- X. H. Hu, J. C. Yu, J. M. Gong, Q. Li and G. S. Li, Adv. Mater., 2007, 19, 2324–2329 CrossRef CAS.
- S. Sasikala and P. Shakkthivel, RSC Adv., 2015, 5, 74408–74415 RSC.
- P. Shakkthivel, S. Sasikala, M. Ramalakshmi, K. Y. Yun and K. Min, Sens. Actuators, B, 2016, 234, 386–394 CrossRef.
- G. K. Pradhan and K. M. Parida, ACS Appl. Mater. Interfaces, 2011, 3, 317–323 CAS.
- S. Sun, L. You, D. Wang, Y. Sun, J. Ma and G. Lu, Sens. Actuators, B, 2011, 156, 368–374 CrossRef.
- G. Sharma and P. Jeevanandam, RSC Adv., 2013, 3, 189–200 RSC.
- H. Yan, J. Zhang, C. You, Z. Song, B. Yu and Y. Shen, Mater. Chem. Phys., 2009, 113, 46–52 CrossRef CAS.
- P. M. Rao and X. Zheng, Nano Lett., 2011, 11, 2390–2395 CrossRef CAS PubMed.
- M. Aronniemi, M. Sainio and J. Lahtinen, Surf. Sci., 2005, 578, 108–123 CrossRef CAS.
- S. Liu, K. Yao, L.-H. Fu and M.-G. Ma, RSC Adv., 2016, 6, 2135–2140 RSC.
- S. K. Apte, S. D. Naik, R. S. Sonawane and B. B. Kale, J. Am. Ceram. Soc., 2007, 90, 412–414 CrossRef CAS.
- C. Wu, P. Yin, X. Zhu, C. OuYang and Y. Xie, J. Phys. Chem. B, 2006, 110, 17806–17812 CrossRef CAS PubMed.
- L. Bao, H. Yangn, X. Wang, F. Zhang, R. Shi, B. Liu, L. Wang and H. Zhao, J. Cryst. Growth, 2011, 328, 62–69 CrossRef CAS.
- Z. H. Jing, S. H. Wu, S. M. Zhang and W. P. Huang, Mater. Res. Bull., 2004, 39, 2057–2064 CrossRef CAS.
- C. L. Snow, C. R. Lee, Q. Shi, J. B. Goates and B. F. Woodfield, J. Chem. Thermodyn., 2010, 42, 1142–1151 CrossRef CAS.
- S. Palchoudhury, W. An, Y. Xu, Y. Qin, Z. Zhang, N. Chopra, R. A. Holler, C. H. Turner and Y. Bao, Nano Lett., 2011, 11, 1141–1146 CrossRef CAS PubMed.
- Y. H. Zheng, Y. Cheng, F. Bao and Y. S. Wang, Mater. Res. Bull., 2006, 41, 525–529 CrossRef CAS.
- S. H. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. X. Li, J. Am. Chem. Soc., 2004, 126, 273–279 CrossRef CAS PubMed.
- A. Pandikumar, G. T. S. How, T. P. See, F. S. Omar, S. Jayabal, K. Z. Kamali, N. Yusoff, A. Jamil, R. Ramaraj, S. A. John, H. N. Limbe and N. M. Huang, RSC Adv., 2014, 4, 63296–63323 RSC.
- A. K. Bhakta, R. J. Mascarenhas, O. J. D’Souza, A. K. Satpati, S. Detriche, Z. Mekhalif and J. Dalhalle, Mater. Sci. Eng., C, 2015, 57, 328–337 CrossRef CAS PubMed.
- Z. Liang, H. Zhai, Z. Chen, H. Wang, S. Wang, Q. Zhou and X. Huang, Sens. Actuators, B, 2016, 224, 915–925 CrossRef CAS.
- S. Qi, B. Zhao, H. Tang and X. Jiang, Electrochim. Acta, 2015, 161, 395–402 CrossRef CAS.
- M. N. Omar, A. B. Salleh, L. H. Ngee and A. A. Tajudin, Anal. Biochem., 2016, 509, 135–141 CrossRef CAS PubMed.
- V. Vinotha, J. J. Wub and S. Anandana, Anal. Methods, 2016, 8, 4379–4390 RSC.
- K. Z. Kamali, P. Alagarsamy, N. M. Huang, B. Hoong Ong and H. N. Lim, Sci. World J., 2014, 2014, 396135–396148 Search PubMed.
- J. J. Jiang and X. H. Du, Nanoscale, 2014, 6, 11303–11309 RSC.
- D. Zhao, G. Yu, K. Tian and C. Xu, Biosens. Bioelectron., 2016, 82, 119–126 CrossRef CAS PubMed.
- L. Jia, Y. Zhou, Y. Jiang, A. Zhang, X. Li and C. Wang, J. Alloys Compd., 2016, 15, 67–174 Search PubMed.
- H. Xu, J. Xiao, L. Yan, L. Zhu and B. Liu, J. Electroanal. Chem., 2016 DOI:10.1016/j.jelechem.2016.04.032.
- Z. Guo, G. Huang, J. Li, Z. Wang and X. Xu, J. Electroanal. Chem., 2015, 759, 113–121 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15290b |
| This journal is © The Royal Society of Chemistry 2016 |