Development of an α-linolenic acid containing soft nanocarrier for oral delivery: in vitro and in vivo evaluation
Mahendra Singha,
Jovita Kanoujiaa,
Pooja Singha,
Chandra B. Tripathia,
Malti Aryaa,
Poonam Parashara,
Vivek R. Sinhab and
Shubhini A. Saraf*a
aDepartment of Pharmaceutical Sciences, Babasaheb Bhimrao Ambedkar University (A Central University), Vidya Vihar, Raebareli Road, Lucknow-226025, U.P., India. E-mail: shubhini.saraf@gmail.com
bUniversity Institute of Pharmaceutical Sciences, Panjab University, Sector-14, Chandigarh-160014 (UT), India
First published on 10th August 2016
Abstract
The oral bioavailability of simvastatin (SIM) a 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitor is about 5%. That may be due to low intestinal permeability and hepatic first pass metabolism (FPM). The objective of the present investigation was to increase the therapeutic efficacy of SIM via developing a soft nanocarrier i.e. a microemulsion to enhance the intestinal permeability in addition to bioavailability. α-Linolenic acid (ALA) was used in the oil phase with Kolliphor EL 40 as surfactant and Transcutol HP as cosurfactant. A microemulsion formulation was developed for the oral delivery of SIM and characterized for physicochemical parameters. The SIM-loaded microemulsion (MES) was investigated for pharmacodynamic and pharmacokinetic parameters to investigate its suitability as a potential drug delivery system for the treatment of Hyperlipidemia in albino Wistar rats. In pharmacodynamic studies, significant differences in parameters were found between the optimized and marketed formulations. Optimized MES showed significantly higher (P < 0.05) Cmax (107.84 ± 8.95 ng ml−1) than marketed tablets (57.65 ± 4.48 ng ml−1). It was found that AUClast obtained from the optimized MES (409.6 ± 22.54 ng h ml−1) was significantly higher (P < 0.01) than the marketed tablet (155.4 ± 12.78 ng h mL−1). The relative bioavailability (Fr) of the optimized formulation was about 263.5% higher than that of the marketed tablets. Optimized MES exhibited no cytotoxicity. Cellular uptake studies confirmed payload delivery to a cellular site (J774.A1 cell line). The results prove that the prepared microemulsion formulation is an improved and effective oral delivery of SIM for the management of lipid levels.
1. Introduction
The Biopharmaceutics Classification System (BCS) is a scientific structure designed for classifying active drug compounds into four classes based on their permeability through the intestine and aqueous solubility. Class-I drugs (high permeability, high solubility) are easily absorbed with a high rate of absorption and a lower rate of excretion. Class-II drugs encompass a high absorption number and a low dissolution number, which implies that these drugs show high permeability and low aqueous solubility. The absorption of this class of drugs is generally slower than that of Class-I drugs and takes a longer duration of time. The bioavailability of this class of drug products is limited by the rate of dissolution. Class-III (low permeability, high solubility) drugs possess a high difference in the rate and extent of drug absorption. Class-IV (low permeability, low solubility) drugs are typically poorly absorbed via the intestinal mucosa, with substantial variation expected in drug absorption.Simvastatin is a BCS class-II drug,1 and its oral absorption is dissolution rate-limited. It is employed to alleviate primary dyslipidemia and hypercholesterolemia. It is a specific and potent competitive inhibitor of HMG-CoA (3-hydroxy-3-methyl-glutaryl coenzyme A) reductase enzyme, which act as a rate-limiting step in cholesterol biosynthesis in the liver.2,3
It also stimulates the hepatic low-density lipoproteins (LDL) receptors, hence increases the breakdown of LDL. Simvastatin, at gastric pH, is stable and remains in a unionized state. However, at intestinal pH, it gets ionized due to which gastrointestinal tract (GIT) absorption is reduced, resulting in low bioavailability. It also has an extensive hepatic first-pass metabolism (FPM) in the liver, which results in low oral bioavailability. The absolute oral bioavailability of simvastatin is ≤5%.4 Around 13% is excreted in urine4 and 60% in feces in unabsorbed form. The half-life of simvastatin is 0.5–3 hours. Half life of a drug might depend on the different factors such as extent of metabolism and genetic variation among individuals. Simvastatin activation requires hydrolysis by carboxyesterases (CYP3A and other enzymes like CYP3A4, CYP2D6 and CYP2C9 present in plasma and liver) which play an important role in its metabolism. The response to simvastatin in different individuals varies depending upon the level of activity of carboxyesterases found in plasma as well as in liver. Therefore, various pharmacokinetic parameters like bioavailability, half-life, and clearance of simvastatin were found to vary. Such an effect is often seen in the statins class of drugs.
As per the data of WHO, cardiovascular diseases (CVDs) are the leading cause of disability and death in developed countries. It has been predicted that CVDs shall become the main cause of mortality in developing countries by 2020. WHO estimates that 17 million people die from CVD annually,5 and that accounts for around 30% of deaths worldwide, including approximately 40% in high-income countries and 28% in low and middle-income countries. Researchers indicated that non-communicable diseases would account for more than three-quarters of deaths worldwide by 2030; in which CVD alone would be responsible for more deaths in low-income countries than infectious diseases.6
Hyperlipidemia is a lipid metabolic disorder (LMD) and a major reason for cardiovascular diseases.7 Nearly twelve million people die every year due to cardiovascular diseases and cerebral apoplexy.8 Instead of CVDs such as atherosclerosis (hardening of blood vessels) and high blood pressure, some other diseases like hypothyroidism, diabetes mellitus, obesity, renal insufficiency (nephrosis-kidney disease) are the secondary diseases which occurs due to hypertriglyceridemia and high total cholesterol level in body.9
Some diseases such as Gaucher disease, Niemann–Pick disease, and Fabry's disease occur due to the accumulation of fat and cholesterol in the spleen, liver, kidney, lungs, bone marrow, brain, autonomic nervous system, eyes and cardiovascular system.10 Hence, it is important to discover better drug delivery to manage triglyceride and total cholesterol levels for better treatment of diseases pertinent to lipid levels.
In recent advancements, nanotechnology has led to the development of nanosized delivery systems to improve the bioavailability hence the therapeutic effectiveness of lipophilic drugs. Lipid-based nanocarriers like microemulsions are beneficial in the enhancement of drug solubility, protection against enzymatic hydrolysis, and increase in absorption due to membrane fluidity and permeability caused by surfactants, hence improvement of bioavailability. Microemulsions are isotropically clear and thermodynamically stable dispersions of two immiscible liquids (oil and water) that get stabilized by an interfacial film of surfactant molecules or surfactant mixture. Research reports suggest that o/w microemulsions (lipid-based nanocarriers) can be used to improve the aqueous solubility, bioavailability and potential delivery system for hydrophobic/poorly water soluble drugs.11,12
The use of α-linolenic acid as an oil phase for microemulsion preparation with synergistic effect of oil in lowering of lipid levels in combination with simvastatin is the novelty of this work. Further, no such study for combined effect of α-linolenic acid and simvastatin microemulsion has been reported till date, to the best of our knowledge.
The major objective of this work was to develop a stable microemulsion system to improve the oral bioavailability of simvastatin and to compare the results of simvastatin-loaded microemulsions with marketed tablet.
For this purpose, firstly the solubility of the drug in different components such as oils, surfactants and co-surfactants was determined. Then, α-linolenic acid (ω-fatty acid) was selected as the oil phase, with Kolliphor EL40 as surfactant and transcutol HP as co-surfactant. Pseudoternary phase diagrams were constructed with the selected components. Then, final microemulsion formulations were prepared from selected pseudo-ternary phase diagrams (Smix ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and evaluated for in vitro release and particle size analysis. Optimized microemulsion formulation was evaluated for ex vivo intestinal permeability and compared with conventional formulations such as oily solution, emulsion, pure drug suspension and marketed tablet, in vivo pharmacodynamic (antihyperlipidemic activity) and pharmacokinetic studies, cell cytotoxicity, cellular uptake and rheology for an effective oral delivery system of simvastatin.
1) and evaluated for in vitro release and particle size analysis. Optimized microemulsion formulation was evaluated for ex vivo intestinal permeability and compared with conventional formulations such as oily solution, emulsion, pure drug suspension and marketed tablet, in vivo pharmacodynamic (antihyperlipidemic activity) and pharmacokinetic studies, cell cytotoxicity, cellular uptake and rheology for an effective oral delivery system of simvastatin.
2. Experimental
2.1 Materials
Simvastatin was obtained as a gift sample from IPCA Pvt. Ltd. (Mumbai, India). Oleic acid (OA), isopropyl myristate (IPM), sesame oil, soyabean oil, Tween 80, Tween 20 were purchased from HiMedia Laboratories (Mumbai, India). 3-[4,5-Dimethyl thiazolyl]-2,5-diphenyltetrazolium bromide (MTT reagent) was purchased from Sigma Chemicals (St. Louis, MO). α-Linolenic acid was purchased from Rolex Chemicals (Mumbai, India). Kolliphor EL40 and Transcutol HP were obtained as gift samples from Gattefosse India (Mumbai, India). All other chemicals used in the study were of analytical grade.2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (% w/w) to get a complete phase boundary of microemulsion region. Pseudoternary phase diagrams (PPD) were constructed using titration method. Each mass ratio was visually checked for clarity and good flow properties of microemulsion and turbidity was noted at the end point of the titration.
9 (% w/w) to get a complete phase boundary of microemulsion region. Pseudoternary phase diagrams (PPD) were constructed using titration method. Each mass ratio was visually checked for clarity and good flow properties of microemulsion and turbidity was noted at the end point of the titration.| Formulation code | Lipid phase (% w/w) | Smix (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (% w/w) 1) (% w/w) |
Water (% w/w) | Particle size and PDI | Drug content (mg ml−1) ± SD | pH | |
|---|---|---|---|---|---|---|---|
| Particle size (nm) (mean ± SD) | PDI (mean ± SD) | ||||||
| MES1 | 10 | 40 | 50 | 149.9 ± 16.8 | 0.315 ± 0.06 | 9.83 ± 0.39 | 6.6 ± 0.09 |
| MES2 | 10 | 50 | 40 | 143.1 ± 15.9 | 0.290 ± 0.08 | 9.59 ± 0.48 | 6.6 ± 0.18 |
| MES3 | 15 | 40 | 45 | 179.6 ± 18.6 | 0.289 ± 0.06 | 9.62 ± 0.53 | 6.4 ± 0.22 |
| MES4 | 15 | 50 | 35 | 157.2 ± 15.7 | 0.261 ± 0.04 | 10.02 ± 0.03 | 6.5 ± 0.14 |
| MES5 | 20 | 40 | 40 | 297.1 ± 30.6 | 0.300 ± 0.05 | 9.93 ± 0.25 | 5.8 ± 0.30 |
| MES6 | 20 | 50 | 30 | 257.4 ± 35.7 | 0.297 ± 0.06 | 10.01 ± 0.08 | 5.9 ± 0.12 |
Conventional formulations like suspension, oily solution and conventional emulsion, containing an equal amount of SIM were also prepared for the sake of comparison. The particle size of SIM was reduced using pestle mortar, and the 2.0% w/v sodium carboxymethyl cellulose (SCMC) solution added. The mixture was made homogeneous and made up to volume (10 mg mL−1 of suspension). For the preparation of the oily solution, SIM was added to α-linolenic acid (oil) and mixed. The emulsion was prepared by weighing and mixing 30% w/w of α-linolenic acid (oil) and 70% w/w of surfactant (a 5% w/v aqueous solution of Kolliphor EL40). The drug (SIM) was then mixed by agitating at room temperature for 10–15 minutes.
Transmission electron microscopy (H-7500, Hitachi made, Tokyo, Japan) was used to carry out a morphological and structural examination of drug-loaded microemulsion formulation on H7500 machine operating at 100 kV capable of point-to-point resolution. Briefly, 0.5 ml droplets of the microemulsion formulation, stained with 1.0% w/v aqueous solution of phosphotungstic acid (PTA), were directly placed on the copper electron microscopy grids. By using different combinations of bright-field imaging scan at increasing magnification power, the surface morphology and structure of the microemulsion was determined.
Albino Wistar rats (200–250 g) were fasted for around 18 hours with free access to water before performing the experiment. Rats were anesthetized by excessive inhalation of ether. For intestinal permeability, after a midline opening in the abdomen, the small intestine was cut out at two positions: one cut at 4 cm distal from the stomach and another cut at the ileocecal junction. The small intestine (entire part) was vigilantly removed and placed in cold Kreb's Ringer Phosphate buffer (KRPb) and solution ceaselessly aerated with the help of an aerator, before use.
Medial jejunal segments of the small intestine (∼6 cm long part) was used for the ex vivo permeation studies in the present experiments (n = 3). This part was cut and then the circular and longitudinal muscle layers removed carefully without damage of the mucosal layer. This tissue segment was washed (6–8 times) with 5 ml KRPb solution each time, one end ligated with silk thread and carefully everted on the glass rod.
Weight (1 g – glass weight) was tied to the ligated end of the everted gut to create an empty gut sac and to avert peristaltic muscular contractions, which could alter the shape and internal volume of the sac. A 1 g weight was used to maintain required conditions and prevent the sac from thinning. Subsequently, the everted gut sac septum acts as a serosal compartment, filled with 2.0 ml of simulated serosal fluid (KRPb solution). Then it was placed in a bath having 45 ml (acts as mucosal fluid) of the test solution (10 mg mL−1 of formulation) constantly bubbled with atmospheric air (at a rate of 15–20 bubbles per minute) with the help of electrical aerator. The bath containing everted sac was enclosed in an external water jacket to maintain the bath temperature at 37 ± 5 °C.
An aliquot of samples was withdrawn from a serosal compartment at predetermined time intervals (up to 5 hours) and replaced with fresh KRPb solution (maintained at 37 ± 5 °C). Drug concentration was determined spectrophotometrically at 238 nm. The ex vivo permeability of simvastatin was calculated with the help of obtained data.15
2.2.9.1 Apparent permeability coefficients (Papp). Papp of simvastatin was determined from mucosal to serosal side according to the equation given:
| Papp (cm s−1) = (dQ/dt)/(A × Co) | (1) |
2.2.10.1 In vivo antihyperlipidemic study. Albino Wistar rats (150–200 g) were taken for this experiment (Institutional Animal Ethics Committee Registration no. BBDNIIT/IAEC/059/2014). The animals were kept in polyacrylic cages maintained under standard conditions of 25 °C ± 2 °C (12 h light/dark cycle) and had free access to standard animal diet and water, ad libitum. For the induction of Hyperlipidemia, animals were fasted for 18 h.
Animals were divided into five groups (n = 6 animals in each group) i.e. Group I to Group V. Triton X-100 (100 mg kg−1) was freshly prepared in physiological saline solution and administered in a single dose of intraperitoneal injection to induce Hyperlipidemia. After 72 h of Triton injection animals received a defined dose of a standard marketed tablet (SIM), and SIM loaded MES4 for seven days orally. Group I – control animal received CMC (0.5% w/v, p.o.), group II – hyperlipidemic control (Triton X-100, treatment), group III – positive control (SIM – 10 mg per kg per day treated), group IV – dummy formulation (Blank MES4, treatment) and group V – test formulation (SIM loaded-MES4 treatment). On the 8th day, blood samples were collected in tubes by retro-orbital sinus puncture, under mild anesthesia. The collected blood samples were centrifuged (2400 rpm for 10 min) to separate serum and used for various biochemical experiments.
Serum total cholesterol (SC), total serum triglyceride (ST), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) levels were calculated using commercial kits from Span Diagnostics Ltd, (Gujarat, India) according to the manufacturer's specifications. LDL/HDL ratio, SC/HDL ratio and atherogenic index (AI) were also calculated.
2.2.10.2 Estimation of HMG-CoA reductase activity. HMG-CoA reductase activity was determined by an indirect method which was simple and reproducible.16 A 10% w/v tissue (liver) homogenate was prepared using arsenate solution (1 g L−1). Fresh liver homogenate (0.5 ml) was mixed with equal volume of perchloric acid (50 ml L−1). After 5 minutes, samples were centrifuged (2000 rpm, 10 min). The mixture was filtered and filtrate (1 ml) was mixed with 0.5 ml of freshly prepared hydroxylamine reagent (2 mol L−1 reagent); pH 5.5 for HMG-CoA and 2 mol L−1 reagent; pH 2.1 for mevalonate. Ferric chloride reagent (1.5 ml) was added after 5 minutes and mixture shaken well. After 10 min absorbance was taken at 540 nm against blank (without homogenate) using double beam UV-visible spectrophotometer (Labtronics LT-2910).
Briefly, in this method liquid–liquid extraction was done using a mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (60
water (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) by gradient HPLC (Waters 2489, with UV-Visible Detector). The separation was done with analytical reverse phase column (Spherosorb C18, 250 × 4.6 mm) with a flow rate of mobile phase 1.0 ml min−1. The mobile phase contained a mixture of 0.025 M sodium dihydrogen phosphate (acidic i.e. pH 4.5)
40) by gradient HPLC (Waters 2489, with UV-Visible Detector). The separation was done with analytical reverse phase column (Spherosorb C18, 250 × 4.6 mm) with a flow rate of mobile phase 1.0 ml min−1. The mobile phase contained a mixture of 0.025 M sodium dihydrogen phosphate (acidic i.e. pH 4.5)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (25
acetonitrile (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 v/v). For analysis of plasma samples, plasma was mixed with mobile phase, vortexed and centrifuged (5000 rpm, 10 min) and extracted supernatant collected in 2 ml centrifuge tube. The extracted supernatant was dried and reconstituted with the mobile phase, filtered through a 0.22 μm membrane filter and then analyzed by HPLC. Obtained data was treated with the help of software (WinNonlin® Software, Version 1.5) and pharmacokinetic parameters obtained.
75 v/v). For analysis of plasma samples, plasma was mixed with mobile phase, vortexed and centrifuged (5000 rpm, 10 min) and extracted supernatant collected in 2 ml centrifuge tube. The extracted supernatant was dried and reconstituted with the mobile phase, filtered through a 0.22 μm membrane filter and then analyzed by HPLC. Obtained data was treated with the help of software (WinNonlin® Software, Version 1.5) and pharmacokinetic parameters obtained.
The relative bioavailability (Fr) of final optimized microemulsion (test) was calculated with respect to marketed tablet (standard) using the equation:
| Fr (%) = AUCtest /AUCstandard × 100 | (2) |
| Cytotoxicity (%) = (absorbance of test/absorbance of blank) × 100 | (3) |
3. Results and discussion
3.1 Solubility studies and component selections
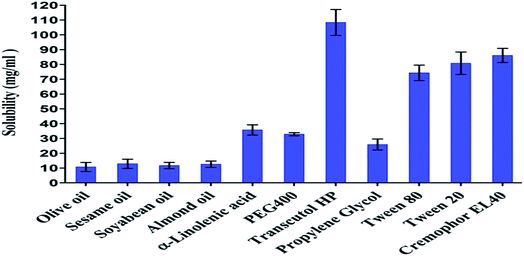
An essential criterion for the selection of components for the development of SIM-loaded microemulsion was the solubility of SIM in the microemulsion components. The prepared microemulsions consisted of oil, surfactants, cosurfactants, drug and aqueous phase. The solubility of pure SIM was performed in various oils, surfactants, and cosurfactants (results are shown in Fig. 1).The solubility of SIM in Kolliphor EL 40 (surfactants, 86.06 ± 4.75 mg ml−1) and Transcutol P (cosurfactants, 108.35 ± 8.77 mg ml−1) and α-linolenic acid (oil phase, 35.72 ± 3.46 mg ml−1) was found higher compared to other vehicles used in solubility analysis. These components were selected respectively, for the preparation of microemulsion formulations resulting in high drug loading capacity. ALA was selected as oil phase since it had significantly higher drug solubility than olive oil, sesame oil, soyabean oil and almond oil. Drug solubility in oils was in the order: α-linolenic acid > sesame oil > almond oil > soyabean oil > olive oil.
The selection of the oil phase i.e. internal lipid phase is the most significant factor since drug solubility in the formulation depends largely on it.18,19 Thus, selection of a suitable oil, surfactant, and cosurfactant which maximizes drug solubility is necessary to get optimal drug loading.20
3.2 Pseudo-ternary phase diagrams
Pseudoternary phase diagrams were constructed with the purpose of studying the association between the phase behavior and the composition of the ME. Phase diagrams also help to decide the concentration series of components for the formation of ME. The pseudo-ternary phase diagrams comprising α-linolenic acid (ALA), Kolliphor EL40, Transcutol HP, and water displayed a region of microemulsion formation (shaded area) at room temperature (as shown in Fig. 2). Finally, phase diagrams of various Smix ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were constructed using ALA as the oil phase, Kolliphor EL40, and Transcutol HP as surfactant mixture (Smix). Smix ratios, 1
1 were constructed using ALA as the oil phase, Kolliphor EL40, and Transcutol HP as surfactant mixture (Smix). Smix ratios, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 2
0 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed less microemulsion region (Fig. 2A and C) than Smix ratio 1
1 showed less microemulsion region (Fig. 2A and C) than Smix ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2B).
1 (Fig. 2B).
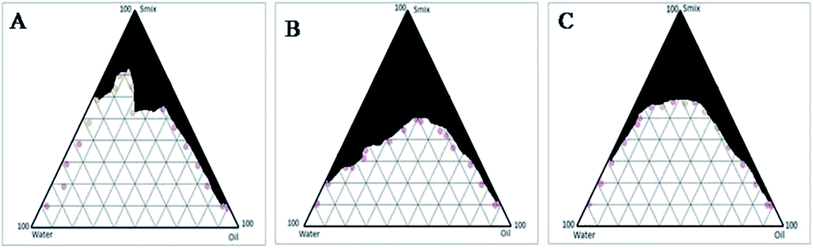 | ||
Fig. 2 Pseudoternary phase diagrams containing Kolliphor EL40 and Transcutol HP (A) Smix (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), (B) Smix (1 0), (B) Smix (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (C) Smix (2 1) (C) Smix (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
That could be owing to less solubilization capacity of oil in the particular ratio of Smix. To minimize potential toxicity of high surfactant content and to avoid gel formation due to the high content of Kolliphor EL40, the Smix ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was selected.
1 was selected.
Further, microemulsion formulation components were selected from the pseudo-ternary phase diagram having largest microemulsion region for a transparent and one phase, low-viscosity microemulsion system.
Microemulsions formed a fine oil-in-water (o/w) system with mere shaking when oil and Smix were added into the aqueous medium. As the free energy involved in forming a microemulsion is very low, the microemulsion formation is spontaneous and thermodynamically stable.21 Since surfactants make a layer around the droplets, hence they lessen the interfacial energy in addition to providing a mechanical barrier to coalescence of the droplets. The visual test measured the evident spontaneity of microemulsion formation.
3.3 Selection and preparation of SIM loaded microemulsion formulations
It revealed through the various pseudo-ternary phase diagrams that the highest microemulsion region is for Smix 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as shown in Fig. 2B. From the selected pseudo-ternary phase diagrams, microemulsions were prepared with different Smix and oil concentration (% w/w) as shown in Table 1 and Fig. 2B. In all cases, Smix concentration varied between 40 and 50% w/w while oil concentration varied between 10 and 20% w/w. Therefore, microemulsion having 40–50% w/w of surfactant component, containing ratios of oil to Smix to water of 10
1 as shown in Fig. 2B. From the selected pseudo-ternary phase diagrams, microemulsions were prepared with different Smix and oil concentration (% w/w) as shown in Table 1 and Fig. 2B. In all cases, Smix concentration varied between 40 and 50% w/w while oil concentration varied between 10 and 20% w/w. Therefore, microemulsion having 40–50% w/w of surfactant component, containing ratios of oil to Smix to water of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 10
50, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 15
40, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, 15
45, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, 20
35, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 20
40 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (% w/w) were selected from phase diagram (Table 1 & Fig. 2B).
30 (% w/w) were selected from phase diagram (Table 1 & Fig. 2B).
Microemulsion MES1 and MES2 contained the same quantities of oil (10% w/w), but Smix concentration changed. It was observed that MES1 showed gel-like viscosity while MES2 displayed a lower viscosity and flowability. This could be attributed to high water content and hydrophilic nature of surfactants (Kolliphor EL40 and Transcutol HP) which swell and form continuous structures when mixed in respective ratios. Other microemulsion formulations i.e. MES3 to MES6 were less viscous with better flow properties. It has been observed that when oil concentration increases and water concentration decreases, less viscous microemulsions having better flow properties are formed.
3.4 Drug and excipients compatibility studies
The compatibility of drug and excipients, used in the microemulsions were performed and characterized through their FTIR spectra analysis. The FTIR spectrum of pure drug SIM has three characteristic peaks at 3550.3, 2963.2, and 1703.2 cm−1 for O–H stretching vibration, C–H vibration and ester stretching vibration and lactone carbonyl functional group, respectively. The FTIR spectrum of prepared formulation also showed three characteristic peaks at 3382.2 cm−1, 2925.4 cm−1, and 1645.5 cm−1. The FTIR spectrum of SIM and prepared formulation were almost similar because of the same functional groups. This indicates that there was no interaction between SIM and the excipients used in the formulation.3.5 Drug content and pH
Drug content of all the prepared MEs formulation was found to be in the range of 9.59 to 10.02 mg ml−1 (Table 1) representing the appropriateness of the system for high entrapment in the internal phase. The drug loading could have been increased in formulations having higher lipid and Smix concentrations. However, since this was the required concentration/dose, a larger quantity was not added. The pH of all MEs was found to be in the range of 5.8 to 6.6 (Table 1), which is suitable for oral delivery. That may also help in reducing the irritation produced upon oral intake of acidic systems.3.6 Globule size, PDI and surface morphology
It was observed that the concentration of excipients taken, especially the lipid: mix surfactant ratio had a direct relationship with the globule size (Table 1).The mean globule size of all the prepared microemulsion formulations containing ALA as oil, Transcutol HP as co-surfactant and Kolliphor EL 40 as a surfactant (Smix in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), were found to be in the range of 143.1 to 297.1 nm, and PDI values varied from 0.261 to 0.315, respectively. Formulations demonstrated low PDI values, indicating uniform droplet size distribution. The smaller the droplet size larger is the surface area available for partitioning of the drug, which may enhance the rate of intestinal absorption of SIM. Lowest particle size was recorded for MES2 (143.1 ± 15.9 nm) with a PDI of 0.290 ± 0.08. Highest particle size was observed for MES5 (297.1 ± 30.6 nm) with a PDI of 0.300 ± 0.05.
1 ratio), were found to be in the range of 143.1 to 297.1 nm, and PDI values varied from 0.261 to 0.315, respectively. Formulations demonstrated low PDI values, indicating uniform droplet size distribution. The smaller the droplet size larger is the surface area available for partitioning of the drug, which may enhance the rate of intestinal absorption of SIM. Lowest particle size was recorded for MES2 (143.1 ± 15.9 nm) with a PDI of 0.290 ± 0.08. Highest particle size was observed for MES5 (297.1 ± 30.6 nm) with a PDI of 0.300 ± 0.05.
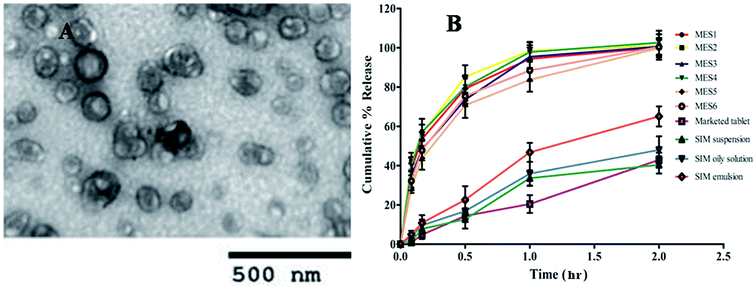
Surface morphology of optimized microemulsion formulation was performed through TEM analysis. Microemulsions revealed spherical globule formation as shown in Fig. 3A. The interphase of oils and Kolliphor EL40 and Transcutol HP displayed a denser region which indicated film formation by Smix, which prevented the globules from coalescence.
3.7 In vitro dissolution study
All the prepared microemulsions (MES1 to MES6), the conventional dosage forms such as SIM suspension, SIM oily solution, SIM emulsion and marketed tablet (10 mg tablet) were subjected to dissolution studies to compare the release profiles of simvastatin. The in vitro release of simvastatin from these dosage forms was evaluated in simulated intestinal fluid (phosphate buffer, pH 6.8); the release percentage of simvastatin from the microemulsion formulations was found significantly higher than that of simvastatin from the conventional dosage form and marketed tablet (Fig. 3B). The release rate of simvastatin from MES1, MES2 MES3, and MES4 was faster than MES5 and MES6. Thus, the release rate of the drug could be modulated through increase or decrease in particle size. It was observed that as the concentration of Smix increased the release rate increased and as the oil concentration increased release rate decreased. MES1 to MES4 released >94%, MES5, and MES6 released >80% and marketed tablet showed 20.46 ± 4.54%, SIM suspension 33.67 ± 3.98%, oily solution 35.92 ± 6.02, emulsion 46.79 ± 4.85% drug release at the end of 1 h in the dissolution medium. A significant increase in percentage drug releases was achieved in the case of microemulsion formulations as compared to conventional formulations. Microemulsion formulation showed complete drug release while conventional formulations exhibited incomplete drug release at the end of 2 h in the dissolution medium. That could be because simvastatin dissolved completely in microemulsion formulations, and possibly displayed faster release due to the small globule size while unsolubilized simvastatin displayed a slower release profile in case of the conventional dosage form.Thus, it was concluded that the presentation of simvastatin at the molecular level in the form of the microemulsions formulation led to enhanced solubilization and increased drug release. This finding also supports the hypothesis that nanosized droplets of microemulsion can increase the release of poorly soluble drugs.22
3.8 Ex vivo permeation study
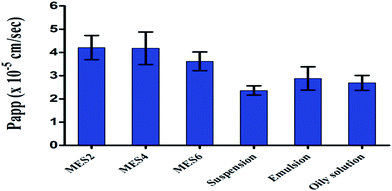 | ||
| Fig. 4 Apparent permeability (Papp, cm s−1) of SIM-loaded formulations through everted rat gut sac after 1 h. | ||
The permeability of simvastatin from microemulsion was found to be significantly higher (P < 0.01) when compared to the drug suspension, drug emulsion and oily solution, indicating the effect of nanosized droplets (Fig. 4). It was observed that as globule size decreases, the permeation of drug increases. A SIM-loaded microemulsion showed Papp 4.20 × 10−5 cm s−1, 4.18 × 10−5 cm s−1 and 3.62 × 10−5 cm s−1 for MES2 MES4 and MES6 respectively after 1 h, while conventional formulations showed a maximum Papp of 2.36 × 10−5 cm s−1, 2.88 × 10−5 cm s−1 and 2.69 × 10−5 cm s−1 from drug suspension, emulsion, and oily solution, respectively. Nanosized globules have better interaction with the biological membrane and are able to permeate through intestinal membranes. Based on the apparent permeability, (Paap) MES4 formulation was selected for rheological behavior, cell cytotoxicity, cellular uptake, in vivo pharmacodynamic and pharmacokinetics studies. Since MES4 contains a higher concentration of oil than MES2 and the selected oil also has some extent of lipid lowering activity,23 therefore MES4 was selected for further studies.
3.9 Rheological behavior
Among the other parameters for the description of MEs, rheology is an important approach to investigate the structural property and obtain information on the storage, handling and pipeline transportation as well stability of MEs.24 The physicochemical evaluation of prepared delivery systems is an essential step in the pre-formulation progression to predict the feasibility of the final products.The rheological analysis showed that the viscosity was low for prepared microemulsion. It appeared to decrease at low shear rates and remained almost constant at higher shear rates as shown in Fig. 5B, while the flow curves confirmed that the ME system revealed a linear relationship between the shear stress and shear rate, which is a characteristic of newtonian flow (Fig. 5A).25
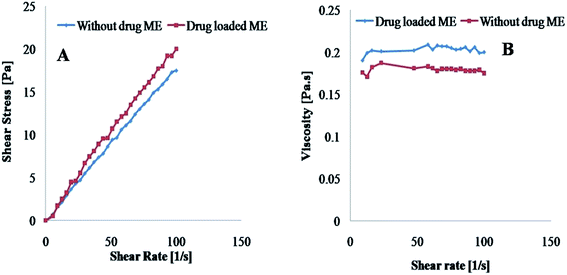 | ||
| Fig. 5 Rheology of prepared optimized microemulsion with and without drug: (A) flow behavior and (B) viscosity with respect to shear rate. | ||
The results confirmed that prepared formulation is discontinuous ME. As reported in the literature, discontinuous MEs display constant viscosity over a wider range of shear rates than bicontinuous MEs.26 As a result of their low viscosity such prepared delivery systems are considered suitable for oral drug delivery.27 The effect of SIM on the microstructures of the MEs was also examined. No change in the linear profile of the flow curves was observed (Fig. 5A), demonstrating that the drug did not influence the flow properties of the prepared microemulsion.
3.10 Cytotoxicity assay
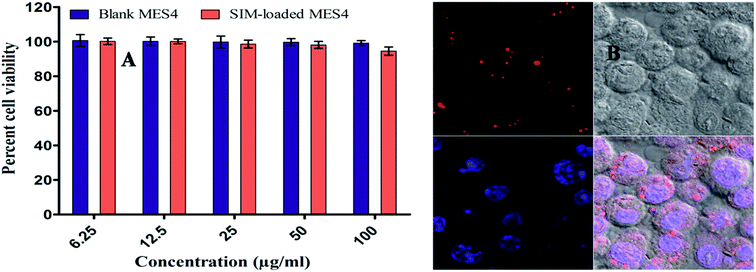
For cell Cytotoxicity, optimized microemulsion (MES4) were suitably diluted and cell toxicity assay performed on the cell lines (J774), at different concentrations of drug loaded microemulsion formulation (i.e. 6.25, 12.50, 25.0, 50.0 and 100.0 μg ml−1). Drug loaded MES4 was found to be sufficiently non toxic where 94.52 ± 2.36% of the cells were found to be viable as compared to placebo MES4 with 99.14 ± 1.42% at concentration 100 μg ml−1 (Fig. 6A). Results indicated nontoxicity and good biocompatibility of the drug loaded microemulsion towards cells and this was confirmed by the cell viabilities (above 90% for all test samples up to 100 μg ml−1) even after 24 h of incubation.3.11 Cellular uptake studies of microemulsions
Few studies have been reported regarding cellular uptake of nano or microemulsions. To explore the distribution of microemulsions in murine macrophages J774 cells, cultured cells were viewed after incubation with optimized microemulsions (labeled by Nile red dye) by confocal fluorescence microscopy (CFM). Confocal microscopy revealed intracellular uptake of the optimized microemulsion in J774 cells. The red channel revealed that microemulsion formulation was majorly co-localized in the cell cytoplasm. Results suggested that the microemulsion efficiently transported the payload to the cellular sites in the cell and not in the nucleus of the cell as shown in Fig. 6B. Various transport pathways to deliver nanoparticles into cells have been reported, such as passive transport, active transport, clathrin or caveolae-mediated pinocytosis, or endocytosis.28 Since, the microemulsion is a nanosized globules delivery system, hence its intracellular uptake could be due to all or one of the mechanism mentioned above as supported through earlier studies.293.12 In vivo pharmacodynamic activity and estimation of HMG-CoA reductase activity (H/M ratio)
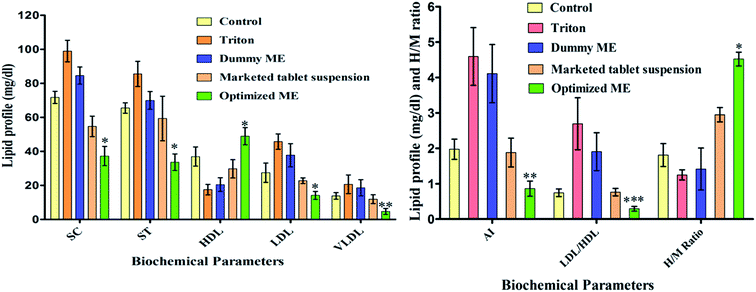
Simvastatin decreases the raised total cholesterol (SC) and total triglyceride (ST) levels in the blood. Simultaneously, it also elevates the high-density lipoprotein (HDL) cholesterol level, which helps the elimination of cholesterol from the peripheral cells and helps its delivery back to the liver.30High plasma concentrations of cholesterol, especially those of low-density lipoprotein (LDL) cholesterol, have been illustrated as one of the prime risk factors for atherosclerotic cardiovascular disease and many other ailments.9,10,31 Therefore, the present study was designed to compare the in vivo hypolipidemic activity of the optimized simvastatin loaded microemulsion (MES4) formulation with the marketed tablet suspension. For induction of hyperlipidemia in rats, Triton was used. A significant increment was found in SC, ST, LDL, VLDL levels while HDL level decreased in Triton treated rats when compared with control rats (without Triton treatment).
In vivo results revealed significant activity (P < 0.001, using one-way ANOVA with Dunnet Multiple comparison tests) towards lipid profiles. An increase in HDL level was found in the optimized MES4 (48.933 ± 5.07 mg dl−1) when compared with marketed tablet (29.77 ± 5.351947 mg dl−1). Significant reduction (P < 0.001) in the SC, ST and LDL levels was observed with optimized MES4 with values of (37.23 ± 5.65 mg dl−1), (33.60 ± 4.88 mg dl−1) and (10.07 ± 3.52 mg dl−1) respectively as compared to marketed tablet having values of (54.67 ± 6.03 mg dl−1), (59.33 ± 13.05 mg dl−1) and (27.13 ± 4.16 mg dl−1) respectively and VLDL significantly reduced (P < 0.01) for optimized ME (3.97 ± 2.35 mg dl−1) as compared to marketed tablet suspension (11.87 ± 2.61 mg dl−1). Optimized ME is a simvastatin-loaded microemulsion (containing ω-3-fatty acid as the oil phase, MES4). Optimized ME (MES4) significantly increased the level of HDL while significantly reducing the levels of SC, ST, LDL and VLDL when compared with simvastatin marketed tablet as shown in Fig. 7. That could be attributed to the solubilization of drug leading to increased absorption of the drug and therefore, improved lipid levels. These results indicate that the prepared microemulsion was efficient in controlling lipid levels as compared to marketed tablet suspension.
Some clinical studies revealed that taking of α-linolenic acid (ω-3-fatty acid) as a supplement alters the serum and tissue triglycerides and free fatty acids levels in fasting and postprandial conditions.23 It was found that omega −3 fatty acids decrease hepatic secretion of triglyceride-rich lipoproteins (VLDL and LDL).32,33 There are various studies which report that when ω-3-fatty acid in combination with simvastatin is administered to hypertriglyceridemia patients, non-HDL cholesterol lowered significantly and also reduced the triglycerides and VLDL levels.33–35
Placebo ME (without SIM) showed some extent of anticholesterolemic activity which could be attributed to its ω-3-fatty acid content.32,33 The combination of ω-3-fatty acid and therapeutic agent (simvastatin) as microemulsion can reduce the risk of cardiovascular diseases via regulation of cholesterol.34,36
Various epidemiological and clinical studies have reported that the total cholesterol (SC)/total high-density lipoprotein (HDL) also known as the atherogenic index (AI) and low-density lipoprotein (LDL)/HDL ratios are indicator of cardiovascular risk and monitor the efficacy of lipid-lowering therapies.37–39
In vivo anti-hyperlipidemic studies revealed a significant (P < 0.01) difference in AI ratios as shown in Fig. 7, when the AI of optimised MES4 (0.76 ± 0.112) was compared with marketed tablet (1.88 ± 0.406) and significant difference (P < 0.05) in LDL/HDL ratios were observed, when optimized MES4 (0.206 ± 0.0703) was compared with marketed (0.91 ± 0.0318) tablet (Fig. 7). It was therefore concluded that optimized MES4 significantly reduces AI and LDL/HDL ratios when compared with marketed tablet. A significant reduction in AI and LDL/HDL ratio with prepared formulation could give better protection from cardiovascular risks.38
H/M assay describes the degree of cholesterol synthesis by measuring the activity of enzyme HMG-CoA reductase.16 The H/M ratio was found to be (1.81 ± 0.32) for control, (1.25 ± 0.14) for toxic, (2.95 ± 0.20) for marketed tablet, (4.52 ± 0.19) for optimized MES4 formulation, and (1.41 ± 0.59) for dummy MES4 respectively. In MES4 formulation treated groups, cholesterol synthesis in liver was significantly lower (high H/M ratio) as compared to marketed tablet treated groups (P < 0.001) as shown in Fig. 7.
3.13 In vivo pharmacokinetic studies
The significant disparity of the factors directing drug absorption in vivo between the microemulsion preparations and marketed tablet were possibly attributed to the following factors: simvastatin is a BCS Class II drug, which implies that although the drug has high permeability, but it has low solubility as a result of low oral bioavailability. Due to low aqueous solubility, absorption is restricted via the dissolution rate of the formulation. Nanosizing/decrease in the particles size is an important factor for improving the oral absorption of this class of drugs.The plasma concentration and time profile curve for simvastatin after oral administration of the marketed tablet suspension (10 mg Tablet) and SIM-loaded optimized microemulsion (MES4) formulation (Fig. 8) and the pharmacokinetic parameters (WinNonlin software) are presented in Table 2.
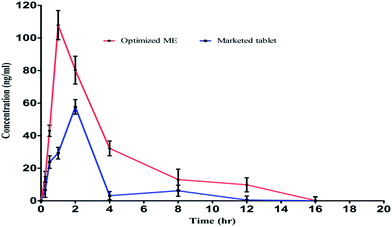 | ||
| Fig. 8 Mean plasma concentration (mean ± SD, n = 6) and time curve of simvastatin after oral administration of microemulsion (MES4) and marketed tablet. | ||
| Pharmacokinetics parameters | Marketed tablet | MES4 formulation |
|---|---|---|
| a P < 0.01 statistically significant difference in AUClast and AUMClast of optimized MES4 as compared to marketed tablet.b P < 0.05 statistically significant difference in Cmax of optimized ME as compared to marketed tablet. | ||
| Tmax (h) | 2.0 | 1.0 |
| Cmax (ng ml−1) | 57.65 ± 4.48 | 107.84 ± 8.95b |
| AUClast (ng h ml−1) | 155.40 ± 12.78 | 409.46 ± 22.54a |
| AUMClast (ng h2 ml−1) | 461.43 ± 30.58 | 1611.56 ± 32.68a |
| MRTlast (h) | 2.97 | 3.94 |
Formulation MES4 revealed significantly higher plasma concentration (Cmax, 107.84 ± 8.95 ng ml−1) (P < 0.05, unpaired t-test with Welch correction) compared to marketed formulation (Cmax 57.65 ± 4.48 ng ml−1). The AUClast for MES4 was found to be 409.46 ± 22.54 ng h ml−1, which was significantly higher (P < 0.01, unpaired t-test with Welch correction) than that of marketed formulations (155.40 ± 12.78 ng h ml−1). AUMClast for MES4 was found to be 1611.56 ± 32.68 ng h2 ml−1, which was significantly higher (P < 0.01) than that of marketed formulations (461.44 ± 30.58 ng h2 ml−1). The enhancement in AUC and Cmax could be due to the fact that drug molecules were absorbed faster from the gastrointestinal tract, due to the reduction in globules size, increased surface area for absorption and also increased dissolution rate.
When Tmax of the optimized MES4 formulation was compared with marketed formulation, Tmax of the optimized formulation was observed to be lesser than marketed formulation which could be attributed to faster dissolution rate and higher Tmax could be observed for marketed formulation due to crystalline nature of the pure drug.40
Area under the curve (AUC) for optimized MES4 showed a 2.635-fold improvement from AUC generated after administering marketed tablet formulation, indicating a significant improvement of simvastatin bioavailability when given orally as microemulsion.41
In addition to the surface area of the microemulsion globules, changes in the permeability of the intestinal mucosa due to the presence of surfactant mixture (Kolliphor EL40 and Transcutol HP) and better permeation of simvastatin across the gastric barrier, as well as a decrease in the interfacial tension between the formulation and lipophilic mucosal layers occurred, hence absorption of simvastatin increased. Other components of the microemulsion formulations i.e. α-linolenic acid may be expected to enhance permeation due to interaction with intestinal lipids making them suitable excipients for such formulations.19
The surfactant and co-surfactant (Kolliphor EL40 and Transcutol P) may have contributed to enhancement in permeability of biomembranes or improved affinity between lipid particles and the gut wall. MES4 may adhere to the gut membrane or enter the intervillar spaces thus extending the time of contact in the gastrointestinal tract and may thus modulate intestinal permeability through apically polarized efflux system, leading to enhanced oral bioavailability.42
When the mean residence time (MRT) of the optimized MES4 was compared with marketed tablet, a significant difference was observed which indicated that their elimination time was comparable. The relative bioavailability of the MES4 increased 263.5% with respect to marketed tablet suspension. Thus, there was about 2.635 fold increase in bioavailability of Simvastatin from MES4 which could be attributed to increased surface area due to nanosizing and improved dissolution rate.
3.14 Histopathological study
Histopathology of liver tissue of control group of rats was distinguished by the absence of Kupffer cell hyperplasia, steatosis, sinusoidal dilatation and congestion with organized hepatic structure (Fig. 9A).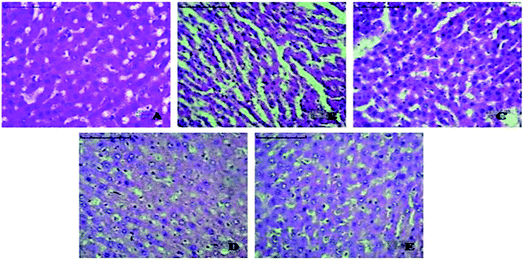 | ||
| Fig. 9 Histopathology of liver A. Control, B. Toxic control, C. Dummy microemulsion, D. Optimized SIM-loaded microemulsion (MES4) and E. Marketed tablet treatment. | ||
While morphological changes (sinusoidal dilatation, necrosis of hepatocytes) were observed in the toxic control group with wide spaces (Fig. 9B), further investigation on liver tissue of rats treated with dummy optimized microemulsion formulation showed some extent of regaining of sinusoidal dilatation and necrosis of hepatocytes compared with toxic control group liver cells. Liver histopathology of rats treated with optimized placebo microemulsion (Fig. 9C) confirmed mild sinusoidal congestion and wide sinusoidal spaces. The sections of rats treated with drug loaded optimized microemulsion (Fig. 9D) showed more recovery of hepatic architecture with preserved parenchymal structures (darkly stained nucleus, no sinusoidal dilatation, and congestion, no necrosis in hepatocytes) than marketed tablet treated rats (Fig. 9E).
4. Conclusions
In recent times, various studies have developed ME for lipid level management with simvastatin but there is no study with ME formulations containing α-linolenic acid as oil phase and simvastatin as a drug. In this experiment, a novel o/w soft-nanocarrier i.e. ME system, for oral delivery of simvastatin has been developed. According to physicochemical, in vitro and in vivo characterization, it may be concluded that simvastatin was incorporated into the ME successfully. Results indicate that developed ME is a promising delivery system for the oral drug delivery of simvastatin for better treatment of lipid levels in hyperlipidemic rats.Finally, it was concluded that the experiments could be prudently extrapolated to develop a novel colloidal soft-nanocarriers containing α-linolenic acid as the oil phase, providing appropriate platform technology(ies) for enhancing the oral bioavailability of other BCS class-II drugs, especially those undergoing extensive hepatic first-pass metabolism. Further, there is a need to develop an in vitro and in vivo correlation between the developed and marketed formulation.
In future, instead of cardiovascular disease treatment, this type of delivery system may also be useful to treat various types of ailments such as diabetes, obesity, hypothyroidism, Gaucher disease, and Niemann–Pick disease, which are associated with lipids level.
Declaration
The authors declare no prospective conflicts of concern with respect to the authorship and/or publication of this article.Acknowledgements
The authors acknowledge the kind support of IPCA pharmaceuticals, Pvt. Ltd., Mumbai and Colorcon Ltd. India (Gattefosse, France), for providing the gift samples of simvastatin and Transcutol HP respectively. Authors also acknowledge the SAIF department, Panjab University, Chandigarh for providing some evaluation facilities for this research work.References
- R. Löbenberg and G. L. Amidon, Eur. J. Pharm. Biopharm., 2000, 50, 3 CrossRef.
- H. Cheng, S. C. Sutton, J. D. Pipkin, G. M. Zentner, J. D. Rogers, J. I. Schwartz, Y. B. Mitchel, K. Grasing, M. S. Schwartz, R. D. L. Amin, L. Liu, D. L. Ebel, A. Coulter, K. Engle, G. A. McClelland, C. Y. Lui and G. S. Rork, Pharm. Res., 1993, 10, 1683 CrossRef CAS.
- C. A. McClelland, R. J. Stubbs, J. A. Fix, S. A. Pogany and G. M. Zentner, Pharm. Res., 1991, 8, 873 CrossRef.
- M. Schachter, Fundam. Clin. Pharmacol., 2005, 19, 117 CrossRef CAS PubMed.
- http://www.who.int/cardiovascular_diseases/resources/atlas/en, accessed February 2016.
- R. Beaglehole and R. Bonita, Lancet, 2008, 372, 1988 CrossRef.
- A. Park, Medicine, 2009, 37, 497 CrossRef.
- T. T. Hlaing and A. Park, Medicine, 2013, 41, 607 CrossRef.
- G. Yuan, K. Z. Al-Shali and R. A. Hegele, Can. Med. Assoc. J., 2007, 176, 1113 CrossRef PubMed.
- http://www.ninds.nih.gov/disorders/lipidstoragediseases/detaillipidstoragediseases, accessed date, March 2016.
- Y. Lu, K. Wu, L. Li, Y. He, L. Cui, N. Liang and B. Mu, Int. J. Nanomed., 2013, 8, 1879 CrossRef PubMed.
- M. Laupheimer, T. Sottmann, R. Schweins and C. Stubenrauch, Soft Matter, 2014, 10, 8744 RSC.
- A. Zvonar, K. Berginc, A. Kristl and M. Gasperlin, Int. J. Pharm., 2010, 388, 151 CrossRef CAS PubMed.
- S. Bandyopadhyay, O. P. Katare and B. Singh, Colloids Surf., B, 2012, 100, 50 CrossRef CAS PubMed.
- I. Legen, M. Salobir and J. Kerc, Int. J. Pharm., 2005, 291, 183 CrossRef CAS PubMed.
- A. V. Rao and S. Ramakrishnan, Clin. Chem., 1975, 21, 1523 CAS.
- G. Carlucci, P. Mazzeo, L. Biordi and M. Bologna, J. Pharm. Biomed. Anal., 1992, 10, 693 CrossRef CAS PubMed.
- A. Palamakula and M. A. Khan, Int. J. Pharm., 2004, 273, 63 CrossRef CAS PubMed.
- C. J. H. Porter, C. W. Pouton, J. F. Cuine and W. N. Charman, Adv. Drug Delivery Rev., 2008, 60, 673 CrossRef CAS PubMed.
- A. S. Narang, D. Delmarre and D. Gao, Int. J. Pharm., 2007, 345, 9 CrossRef CAS PubMed.
- D. Q. M. Craig, S. A. Barker, D. Banning and S. W. Booth, Int. J. Pharm., 1995, 114, 103–110 CrossRef CAS.
- F. S. Nielsen, K. B. Petersen and A. Mullertz, Eur. J. Pharm. Sci., 2008, 69, 553 CAS.
- M. S. Weintraub, R. Zechner, A. Brown, S. Eisenberg and J. L. Breslow, J. Clin. Invest., 1988, 82, 1884 CrossRef CAS PubMed.
- R. Pal, Curr. Opin. Colloid Interface Sci., 2011, 16, 41 CrossRef CAS.
- J. L. Feng, Z. W. Wang, J. Zhang, Z. N. Wang and F. Liu, Colloids Surf., A, 2009, 339, 1–6 CrossRef CAS.
- D. P. Acharya and P. G. Hartley, Curr. Opin. Colloid Interface Sci., 2012, 17, 274 CrossRef CAS.
- M. J. Lawrence and G. D. Rees, Adv. Drug Delivery Rev., 2012, 64, 175 CrossRef.
- H. Y. Nam, S. M. Kwon, H. Chung, S. Y. Lee, S. H. Kwon, H. Jeon, Y. Kim, J. H. Park, J. Kim, S. Her, Y. K. Oh, I. C. Kwon, K. Kim and S. Y. Jeong, J. Controlled Release, 2009, 135, 259 CrossRef CAS PubMed.
- X. You, Q. Xing, J. Tuo, W. Song, Y. Zeng and H. Hu, Int. J. Pharm., 2014, 471, 276 CrossRef CAS PubMed.
- https://www.merck.com/product/usa/pi_circulars/z/zocor/zocor, accessed January 2015.
- O. L. Erukainure, J. A. Abovwe, A. S. Adefegha, R. U. Egwuche and M. A. Fafunso, Exp. Toxicol. Pathol., 2011, 63, 657 CrossRef PubMed.
- P. Bordin, O. Bodamer and S. Vaenkatesan, Eur. J. Clin. Nutr., 1998, 52, 104 CAS.
- D. C. Chan, G. F. Watts, P. H. Barrett, L. J. Beilin and T. A. Mori, Clin. Chem., 2002, 48, 877 CAS.
- P. Barter and H. N. Ginsberg, Am. J. Cardiol., 2008, 102, 1040 CrossRef CAS PubMed.
- M. H. Davidson, S. A. Stein, H. E. Bays, K. C. Maki, R. T. Doyle, R. A. Shalwitz, C. M. Ballantyne and H. N. Ginsberg, Clin. Ther., 2007, 29, 1354 CrossRef CAS PubMed.
- B. J. Meyer, T. Hammervold, A. C. Rustan and P. R. Howe, Lipids, 2007, 42, 109 CrossRef CAS PubMed.
- I. Lemieux, B. Lamarche, C. Couillard, A. Pascot, B. Cantin, J. Bergeron, G. R. Dagenais and J. P. Després, Arch. Intern. Med., 2001, 161, 2685 CrossRef CAS PubMed.
- J. Millan, X. Pinto and A. Munoz, et al., Vasc. Health Risk Manage., 2009, 5, 757 CAS.
- M. Mahto, B. Chakraborthy, S. H. Gowda, H. Kaur, G. Vishnoi and P. Lali, Indian J. Clin. Biochem., 2012, 27, 284 CrossRef CAS PubMed.
- E. Merisko-Liversidge, G. G. Liversidge and E. R. Cooper, Eur. J. Pharm. Sci., 2003, 18, 113 CrossRef CAS PubMed.
- G. Sharma, K. Wilson, C. F. van der Walle, N. Sattar, J. R. Petrie and M. N. V. Ravi Kumar, Eur. J. Pharm. Biopharm., 2010, 76, 159 CrossRef CAS PubMed.
- L. Hu, H. Wu, F. Niu, C. Yan, X. Yang and Y. Jia, Int. J. Pharm., 2011, 420, 251 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |