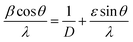DOI:
10.1039/C6RA15119A
(Paper)
RSC Adv., 2016,
6, 82484-82495
Hydrothermal synthesis, structural and luminescent properties of a Cr3+ doped MgGa2O4 near-infrared long lasting nanophospor
Received
10th June 2016
, Accepted 22nd August 2016
First published on 23rd August 2016
Abstract
A novel long lasting phosphor MgGa2O4:Cr3+ was successfully prepared by a hydrothermal method and a solid state reaction method. The hydrothermal method provides nanoparticles of the range 10–70 nm. XRD, FTIR spectroscopy, HRTEM, FESEM, photoluminescence, UV-VIS spectroscopy, and phosphorescence decay measurements were utilized to characterize the phosphor. MgGa2O4 possesses a cubic phase and the Fd3m space group. It crystallizes in a normal spinel structure with Mg2+ ions in tetrahedral coordination and Ga3+ ions in octahedral coordination. The phosphor showed three excitation bands at 225 nm, 445 nm and 558 nm. Upon UV excitation at 225 nm, the material exhibited an emission band from 600–800 nm peaking at 707 nm corresponding to the 2E(2G) → 4A2(4F) spin forbidden transition of the distorted Cr3+ ions in the MgGa2O4 host. Three deconvoluted peaks at 675 nm, 707 nm and 733 nm were found in phosphorescence emission spectra. The decay curve shows that the sample prepared by the solid state reaction method undergoes a sharper decay process than the samples prepared by the hydrothermal method. Slow decay was observed for the sample with lowest concentration. A significant afterglow signal was observed for 1 hour when the sample was excited at 225 nm for 5 minutes. This nanophosphor with an emission band at 600–800 nm can be suitable for in vivo bio imaging application purposes.
1. Introduction
Long persistent phosphor materials are unique kinds of material which can absorb, store and release energy. When these materials are excited by sunlight or any other external energy source, then free electrons and holes are produced which are trapped by the defects present in the material. These charges are de-trapped when the energy difference between the trapping level and host band is close to room temperature energy. This de-trapping of charge carriers can prolong the emission.1,2 Long Lasting Phosphorescence (LLP) is a phenomenon in which emission in the visible or NIR range is observed for a long time after the excitation has ceased.3,4 In the past few decades, these phosphor materials have gained much attention among researchers because of their potential applications in a wide variety of fields such as security applications, decoration, detection of high energy rays, traffic signals, dials and displays, storage media, fibre optic thermometers, in vivo and in vitro bioimaging.5,6 These materials when excited by UV or visible light exhibit emission in the visible to near-infrared range. Among these, NIR long lasting phosphors have received much more attention due to their photoluminescence emission in the range of the biological optical window (600–1000 nm). So these materials can uniquely be used as probes for in vivo imaging, and can be excited externally before injecting into the small animal body which prevents the autofluorescence from the animal body resulting a high signal to noise ratio.7,8 Thus for advanced optical imaging in deep tissues, cells with high resolution and small disturbance, these phosphor materials are very useful.
For NIR photoluminescence, Cr3+ is a favourable luminescent centre in solids because of its narrow emission band near 700 nm due to spin forbidden transition 2E(2G) → 4A2(4F) or a broadband emission (600–1000 nm) due to spin allowed transition 4T2 → 4A2 which depends on the crystal field environment of the host lattices.9,10 Thus, Cr3+ can widely be used as a dopant into various host materials such as gallate AGa5O12 [A = Y, Gd, La];11 LiGa5O8; gallogermanates M3Ga2Ge4O14 [M = Sr or Ca] and La3Ga5GeO14.12–14 Since Cr3+ ions have the ability to substitute Ga3+ ions in distorted octahedral sites, these hosts provide suitable crystal field environment for dopant Cr3+ to achieve NIR emission. Inspired by this, a series of Cr3+ doped LiGa5O8, ZnGa2O4 (ref. 15 and 16) have been reported. Cr3+ doped lanthanum gallogermanate achieved persistent NIR luminescence with a persistent time more than 1 hour.17 Basavaraju et al. have reported Cr3+ doped MgGa2O4 prepared by high-temperature solid-state reaction method.18 But most of the synthesized materials are in a bulk ceramic form which is not suitable for practical biological application purposes. Almost in all long persistent phosphors it is found that after injecting into the animal body there is a decrease in luminescence intensity and in most cases it is required to re-excite the injected materials after its luminescence vanishes which limit the use of this long persistent phosphor for in vivo imaging.19 For this reason ZnGa2O4:Cr3+ was prepared via a hydrothermal route as reported by Zhanjun et al.20 For bioimaging application purposes the material must be nanocrystalline and biocompatible, i.e., they must have the comparable with the size of the biomolecules they are labelling.21,22
Herein, we report the synthesis of Cr3+ doped MgGa2O4 by hydrothermal method. In this synthesis method, the chemical reaction of substances occurs in a sealed heated Teflon-lined autoclave above ambient temperature and pressure. This technique is used to prepare nanoparticles. We have prepared MgGa2−xO4:xCr3+ persistent luminescent nanoparticles with 10–70 nm range and intense PL properties. We have also prepared it by the high-temperature solid state reaction method as a reference and comparison with the nanoparticles prepared by hydrothermal method.
2. Experimental
2.1. Sample preparation
The MgGa2−xO4:xCr3+ (x = 0, 0.25, 0.50, 1 mol%) samples were synthesized by hydrothermal route. The stoichiometric amounts of Mg(NO3)2·6H2O (A.R., Loba Chemie); Ga2O3 (A.R., Alfa Aesar, 99.99%); Cr(NO3)3·9H2O (A.R., Loba Chemie) were taken as raw materials. Ga(NO)3 solution was prepared from Ga2O3 by dissolving it in few mL concentrated HNO3 and heated at around 130 °C for 10 hours to remove excess HNO3. The precursor solutions of Mg(NO3)2·6H2O; Ga(NO)3 and Cr(NO3)3·9H2O were mixed and stirred vigorously. Then NH4OH solution was added in a quick manner to reach a pH at around 9–9.5. The metal hydroxide precursor was then transferred into a Teflon-lined autoclave and sealed. It was followed by a hydrothermal reaction which occurred at 220 °C for 10 hours. After that, the sample was naturally cooled until reaching room temperature. Then the solution was washed several times with ethanol and deionized water and filtered. After filtration, the sample was dried at 80 °C for 3 hours. Then the sample was grinded to get fine powdered sample. After that, some portion of the sample was kept for as-synthesized sample and the rest was annealed at 700 °C for 2 hours in an electrical tube furnace.
In solid state reaction method, the stoichiometric amount of Ga2O3 (A.R., Alfa Aesar, 99.99%), MgCO3 (A.R., Loba Chemie) and CrO3 (A.R., Alfa Aesar Puratronic, 99.97% pure) were mixed in an agate mortar in ethanol medium and grinded for 1 hour. Then the mixed powder was transformed into an alumina crucible and kept in a high-temperature furnace at 1300 °C for 5 hours. Then the sample was naturally cooled and after reaching room temperature, the sample was again grinded to obtain finely powdered sample.
2.2. Characterization
The X-ray diffraction patterns of the samples were recorded for a broad range of 2θ (10° < 2θ < 90°) on a Panalytical high-resolution XRD with Cu Kα = 0.15406 nm. The microstructures of the prepared phosphors were examined by a field emission scanning electron microscope (FESEM Supra-55 Germany). The morphology and sizes of the prepared samples were analysed by the high-resolution transmission electron microscope (HRTEM) using JEOL JEM-2100 HRTEM. SAED and EDAX were also carried out. For HRTEM analysis, a small amount of the sample was dispersed in acetone and then treated with ultrasonic vibration and after ultrasonication, a drop of it was imposed in a carbon coated copper grid. The FTIR spectra (KBr pellet) were recorded on an FTIR spectrum RX 1 (Perkin Elmer) in the range 400–4000 cm−1. The room temperature photoluminescence excitation and emission spectra were recorded on a Hitachi FL 2500 fluorescence spectrophotometer with a xenon lamp of power 150 W. The decay kinetics and room temperature time-resolved phosphorescence emission were measured by Agilent Cary Eclipse Fluorescence Spectrophotometer in the lifetime and phosphorescence mode. The afterglow decay curve was carried out in the kinetic-chemo luminescence mode by Agilent Cary Eclipse Fluorescence Spectrophotometer. Before measuring the afterglow, the sample was irradiated with pulsed xenon lamp at 225 nm for 5 minute equipped in the same instruments. For the comparison of excitation and emission intensities of the prepared samples, all the measurements were done at same instrumental parameters such as same excitation wavelength and power, same excitation and emission slits. The reflectance spectra were also recorded by using an Agilent Cary-500 UV-Vis-NIR double beam spectrophotometer.
3. Results and discussions
3.1. Structural and morphological studies
3.1.1. XRD analysis. The XRD patterns of MgGa2O4:Cr3+ for as-synthesized, annealed and solid state samples are as shown in Fig. 1. The XRD patterns of the samples match well with the standard JCPDS-010-0113 pattern with cubic phase and Fd3m space group. It indicates high purity and crystalline behaviour of the MgGa2O4:Cr3+ nanoparticles in this work. No change in crystal structure of the host MgGa2O4 is observed due to the incorporation of dopant Cr3+. It is because the ionic radius of Cr (0.52 Å) and Ga (0.62 Å) are nearly similar. So Ga site can be replaced by Cr3+ ion. Therefore, a small amount of Cr3+ doping does not have a significant influence on the crystal structure of the host. The lattice parameters (a = b = c) and volumes were calculated using Unit Cell Win Software. The results obtained are shown in Table 1.
 |
| | Fig. 1 Powder XRD patterns of as-synthesized, annealed and solid state reaction method prepared samples of MgGa2O4:Cr3+. | |
Table 1 Lattice parameters of annealed MgGa2O4:0.25 mol% Cr3+, as-synthesized MgGa2O4:0.25 mol% Cr3+ and solid state reaction prepared MgGa2O4:Cr3+ phosphor
| Parameters |
As-synthesized |
Annealed |
Solid state reaction |
| a |
8.26225 Å |
8.28657 Å |
8.26519 Å |
| V |
564.0205 Å3 |
569.0149 Å3 |
564.6226 Å3 |
The typical diffraction peaks were obtained at 18.38°, 30.52°, 35.97°, 43.69°, 54.23°, 57.82°, 63.53°, 75.23° and are identified as (111), (220), (311), (400), (422), (511), (440), (533) planes respectively. The crystallite size of the samples was calculated by Debye–Scherrer formula23
where
β is the full width at half maxima,
K (shape factor) = 0.9 and
λ (wavelength of X-ray) = 0.154 Å.
The crystallite size was also calculated from Williamson–Hall relation given by,
where
ε is the microstrain present in the crystal and
D is the crystallite size.
Fig. 2 shows the Williamson–Hall plots. The results obtained are shown in
Table 2. The range of the crystal sizes was in between 5 and 16 nm with average 13.62 nm as obtained from Debye–Scherrer formula for the as-synthesized and annealed MgGa
2O
4:0.25 mol% Cr
3+ sample prepared by hydrothermal method whereas from Williamson–Hall plot it was found to be 16.65 and 16.8 nm respectively. The crystallite sizes for the sample prepared by solid state reaction were found to be in the range of 34–47 nm as obtained from Debye–Scherrer formula whereas from Williamson formula it was found to be 88.89 nm. Due to heat treatment, the diffraction peaks were found to be sharper, and FWHM of diffraction peaks were decreased when the sample was annealed.
24 In solid state synthesis as the reaction occurred in high temperature, so crystallite size was very large compared to the other samples prepared by hydrothermal method.
 |
| | Fig. 2 Williamson–Hall plot of as-synthesized, annealed and solid state reaction method prepared samples of MgGa2O4:Cr3+. | |
Table 2 Comparison of crystallite size as obtained from Debye–Scherrer and Williamson–Hall formula
| Sample |
Crystallite sizes (D) |
| Debye–Scherrer (nm) |
Williamson–Hall (nm) |
| As-synthesized |
5–16 |
16.65 |
| Annealed |
11–16 |
16.08 |
| Solid state reaction |
35–48 |
88.98 |
3.1.3. FTIR study. The FTIR spectra of as-synthesized, annealed, undoped and solid state reaction prepared MgGa2O4:0.25 mol% Cr3+ sample were also studied and results obtained are presented in Fig. 7. The absorption band obtained at 476 cm−1 and 636 cm−1 were due to Ga–O and Mg–O stretching bands respectively.20,26 The small shift of the peak positions of these samples was due to the doping effect of Cr3+ ion. The absorption band at 1085 cm−1 was attributed to the C–H bending. The absorption bands at 1385 cm−1 and 1631 cm−1 were due to N–O stretching and N–H bending mode respectively.27 The band obtained at 2926 cm−1 was due to C–H stretching. The absorption band at 3447 cm−1 was attributed to the O–H stretching vibration.28 It is also to be observed that the absorption peaks due to N–O stretching, N–H bending and –OH group decreased in the annealed and solid state method sample because of heat treatment. The peak at 1385 cm−1 is noticed to be very strong in as synthesized sample and as the samples were annealed, N–O stretching diminished which results very weak peak at 1385 cm−1 for annealed samples. The peaks due to Ga–O and Mg–O stretching band in the annealed samples were also very sharp which indicates the good crystal quality and phase purity of the samples.29 As for doped sample, the doping concentration was very low, so no peaks were found for the incorporation of the dopant ion. But in comparison to the undoped sample, the Zn–O and Mg–O stretching bands are less sharp for doped sample. It is to be mentioned that the presence of N–H, C–H, O–H band decreases the luminescence intensity since these defects present in the surface of the particles, act as a source of quencher on luminescence intensity.30 The luminescence intensity can be effectively increased after removing these ions via high-temperature heat treatment. When the samples were annealed at 700 °C the N–H, N–O and O–H bands decreased which in turn increased the luminescence intensity. The FTIR study confirmed the presence of less impurity in the sample and its suitability for PL study.
 |
| | Fig. 7 FTIR spectra of undoped, as-synthesized, annealed and solid state reaction prepared MgGa2O4:Cr3+ phosphor. | |
3.2. Optical properties
3.2.1. UV-visible diffuse reflectance study. UV-visible diffuse reflectance analysis was carried out on the prepared samples in order to calculate the optical band gap. The diffuse reflectance spectra of the samples were shown in Fig. 8. The diffuse reflectance spectra were recorded at room temperature in the range of 200–800 nm wavelengths. The spectrum shows an absorption edge at 227 nm corresponding to the band gap of nanophosphor which can also serve as the absorption wavelength of this particular nanophosphor. These absorption bands correspond to the absorption transitions from the ground state 4A2 to the excited 4T1, 4T2 states of the Cr3+ ion (d–d transition of Cr3+).31,32
 |
| | Fig. 8 Diffuse reflectance spectra of undoped, as-synthesized, annealed and solid state reaction prepared MgGa2O4:Cr3+. | |
From the DR spectrum, the band gap of MgGa2O4:Cr3+ nanophosphor were determined by using the Kubelka–Munk theory. The absorption spectrum of the phosphor was transformed to Kubelka–Munk function33 given as
where
R is the diffuse reflectance of the spectrum,
K is the absorption coefficient and
S is the scattering coefficient.
The well known Tauc relation relates the direct band gap (Eg) and linear absorption coefficient (α)
hν is the energy of light and
c is constant. The diffuse reflectance spectrum is converted into Kubelka–Munk function by substituting
F(
R∞) in place of
α.
R∞ represents the reflectance of the infinitely thick sample with respect to a reference at each wavelength,
A is a constant.
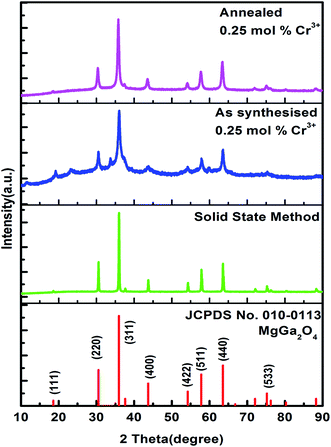
hν is in eV and its relationship to the wavelength λ (in nm) becomes 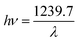 .
.
A line is drawn tangent to the point of inflection on the curve. The value of hν at the point of intersection of the tangent line and the horizontal axis is the band gap (Eg) value. Fig. 9 shows the Kubelka–Munk plot with band gap value of (a) as-synthesized MgGa2O4:0.25 mol% Cr3+ (b) annealed MgGa2O4:0.25 mol% Cr3+ (c) undoped MgGa2O4 (d) solid state reaction prepared MgGa2O4:0.5 mol% Cr3+ phosphor. The band gap energy of the samples increases as the doping concentration increases due to the Burstein–Moss effect34 which states that as the doping concentration increases Fermi level in the conduction band of a degenerate semiconductor shifts upward which leads to the increase of the band gap energy. The band gap value was decreased when the sample was annealed. This is due to the property of semiconductor that the energy band gap value tends to decrease as the temperature increases. Main reason for this fact is that when thermal energy is increased the amplitude of the atomic vibration increases leading to the increase of the interatomic spacing.35 As the interatomic spacing increases, the potential seen by the electron decreases which in turn reduces the size of the band gap.
 |
| | Fig. 9 Kubelka–Munk plot for determination of band gap of (a) as-synthesized MgGa2O4:0.25 mol% Cr3+ (b) annealed MgGa2O4:0.25 mol% Cr3+ (c) undoped MgGa2O4 and (d) solid state reaction prepared MgGa2O4:Cr3+. | |
3.2.2. Photoluminescence studies. The room temperature photoluminescence excitation and emission spectra of the MgGa2O4:0.25 mol% Cr3+ is presented in Fig. 10. The excitation peaks were found at 226 nm, 442 nm and 558 nm corresponding to the 4A2(4F) → 4T1(4P), 4A2(4F) → 4T1(4F), 4A2(4F) → 4T2(4F) spin allowed Cr3+ d–d transitions respectively. The excitation spectra have a maximum absorption band at 225 nm and minimum at 558 nm. The intensity and position of these bands suggest that Cr3+ is in octahedral symmetry. A broad photoluminescence emission spectra were obtained in the range of 600–800 nm originating from the 4T2 → 4A2 spin allowed transition of Cr3+. Fig. 11 shows the PL emission spectra of annealed MgGa2O4:x mol% Cr3+ (x = 0.25, 0.50, 1) and solid state reaction prepared MgGa2O4:0.50 mol% Cr3+ phosphor. The emission peak was obtained at 707 nm corresponding to the 2E(2G) → 4A2(4F) spin forbidden transition of the distorted Cr3+ ions in the MgGa2O4 host when excited with wavelength λexc = 225 nm.36 Cr3+ ions belong to the d3 configuration. 4F state is considered as ground state and 4P, 2G states are excited free electron states. Under the influence of octahedral symmetry field, these states split into quartet and doublet terms such as 4A2, 4T2, 4T1, 2E, 2T1, 2T2, 2A1 etc. According to Hund's rule 4A2 is the lowest state. In a wide variety of oxide based phosphor, the Cr3+ ions are invariably oxygen coordinated with six nearest neighbours which is possible in pure octahedral or distorted octahedral symmetry site.37,38
 |
| | Fig. 10 PL excitation spectrum monitored at 707 nm emission and emission spectra excited at different excitation band 225 nm, 445 nm, 558 nm respectively of annealed MgGa2O4:0.25 mol% Cr3+ phosphor. | |
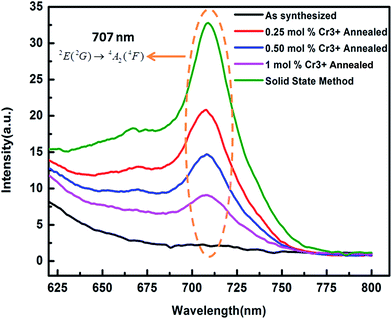 |
| | Fig. 11 PL emission spectra excited at 225 nm of annealed MgGa2O4:x mol% Cr3+ (x = 0.25, 0.50, and 1) and solid state reaction prepared MgGa2O4:Cr3+ phosphor. | |
3.2.3. Phosphorescence emission study. Fig. 12a shows the room temperature phosphorescence emission spectra with λexc = 225 nm and delay time 5 ms of as-synthesized MgGa2O4:0.25 mol% Cr3+, annealed MgGa2O4:x mol% Cr3+(x = 0.25, 0.50, 1) and solid state reaction prepared MgGa2O4:0.5 mol% Cr3+ phosphor. It shows broad emission bands within 600–800 nm originating from spin allowed transition 4T2 → 4A2 of Cr3+. Fig. 12b shows the deconvoluted phosphorescence emission spectra of the annealed MgGa2O4:0.25 mol% Cr3+ with delay time 5 ms. The emission spectra show three distinguishable crests at wavelengths 675 nm, 707 nm, and 733 nm because of spin-forbidden transition 2E(2G) → 4A2(4F) of Cr3+ in a regular octahedral environment (R lines) or in the distorted octahedral environment (N lines). As MgGa2O4 has a partially inverted spinel structures i.e. some of the Mg2+ ions occupy octahedral sites and some of the Ga3+ ions occupy tetrahedral site, so the transition of Cr3+ will be more likely in the distorted octahedral environment.18,39 The time-resolved phosphorescence emission spectra for different delay times also have been done on this sample and shown in Fig. 13. The maximum delay time at which the phosphorescence emission was recorded was 1000 ms and still it showed a very small signal peaking at 707 nm. The phosphorescence intensity decreased with increasing delay time.
 |
| | Fig. 12 (a) Phosphorescence emission spectra of as-synthesized MgGa2O4:0.25 mol% Cr3+, annealed MgGa2O4:x mol% Cr3+ (x = 0.25, 0.50, 1) and solid state reaction prepared MgGa2O4:Cr3+ phosphor and (b) deconvoluted phosphorescence emission spectra of the annealed MgGa2O4:0.25 mol% Cr3+ with delay time. | |
 |
| | Fig. 13 Time resolved phosphorescence emission spectra for different delay times of annealed MgGa2O4:0.25 mol% Cr3+. | |
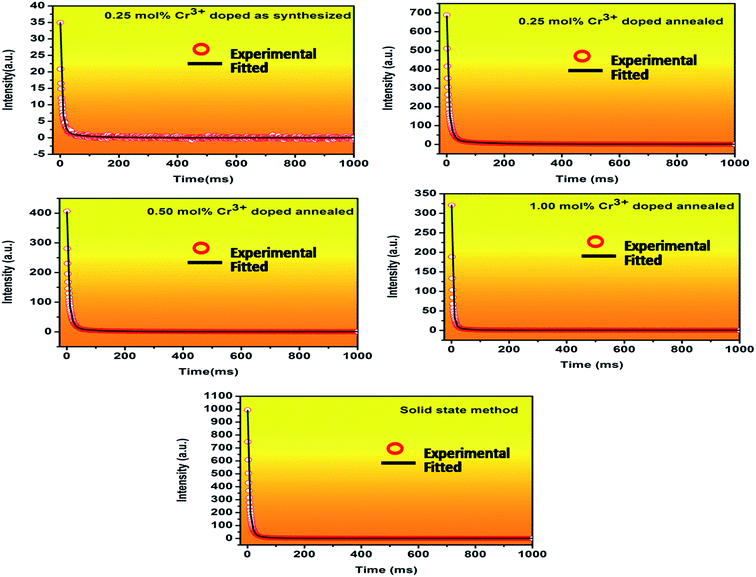
3.2.4. Decay kinetic study. Fig. 14 shows the decay curve of Cr3+ doped MgGa2O4 samples recorded for transition at 707 nm of Cr3+ ion (λexc = 225 nm). The decay curve of the samples was collected in the range of 0–1000 ms and were fitted and analysed by an empirical triple exponential equation given as40
| I = I0 + A1e−t/τ1 + A2e−t/τ2 + A3e−t/τ3 |
where I represent luminescence intensity; I0 is the initial intensity at t = 0; A1, A2 and A3 are constants; τ1, τ2 and τ3 are decay times for the exponential components. The decay parameters are shown in Table 3.
 |
| | Fig. 14 Decay curves of as-synthesized MgGa2O4:0.25 mol% Cr3+, annealed MgGa2O4:x mol% Cr3+ (x = 0.25, 0.50, 1) and solid state reaction MgGa2O4:Cr3+ phosphor monitored at 707 nm emission. | |
Table 3 Decay parameters of as-synthesized MgGa2O4:0.25 mol% Cr3+, annealed MgGa2O4:0.25 mol% Cr3+, annealed 0.5% mol% Cr3+, annealed 1% mol% Cr3+ and solid state reaction prepared MgGa2O4:Cr3+ phosphor
| Samples |
τ1 (ms) |
τ2 (ms) |
τ3 (ms) |
| As-synthesized 0.25 mol% Cr3+ |
0.7817 |
8.572 |
76.46 |
| Annealed 0.25% mol% Cr3+ |
1.817 |
10.173 |
115.233 |
| Annealed 0.5% mol% Cr3+ |
1.259 |
9.261 |
75.31 |
| Annealed 1% mol% Cr3+ |
1.007 |
5.944 |
44.91 |
| Solid state reaction |
1.635 |
7.569 |
46.09 |
From the fitting results, it can be concluded that there are three decay processes. The persistent intensity undergoes a sharp decay process at the very beginning. When the ground state electrons are excited to the higher states some of them simultaneously undergoes to the ground state give the radiative emission at 707 nm followed by some non-radiative transitions between the excited states. τ1 is the time period for this rapid decay process. The rest electrons are captured by the trap levels and undergo slow decay processes. τ2 and τ3 are the time periods for the decay from the shallow and deep traps respectively. The detailed process is discussed in the long persistence mechanism part. For annealed samples as the doping concentrations were increased the values of decay parameters decreased. For this reason, MgGa2O4:0.25 mol% Cr3+ was chosen for further afterglow decay measurement. The sample was excited at 225 nm for 5 minutes and emission wavelength was monitored at 707 nm emission, and then afterglow was recorded as shown in Fig. 15. Significant LLP signal was observed even after 1 hour. This result is very much desired for the application in bioimaging as a probe due to its small particle size and long afterglow characteristics.
 |
| | Fig. 15 Afterglow decay curve of the annealed MgGa2O4:0.25 mol% Cr3+ monitored at 707 nm emission after ceasing off the excitation at 225 nm for 5 min. | |
3.2.5. Long persistence mechanism. We propose a mechanism for NIR persistent luminescence phenomenon at room temperature. The proposed mechanism is shown in a schematic diagram in Fig. 16a and (b). When the material is excited by UV light, the electron trapping and detrapping recombination processes in MgGa2O4:Cr3+ occur via conduction band or through quantum tunnelling processes. Under ultra-violet light excitation at 225 nm, the ground state 4A2 electrons of Cr3+ ions are excited to the higher energy states 4T1 which lie near the conduction band (process 1, Fig. 16a). Then some of the electrons simultaneously undergo some non-radiative transition to the 1st excited state 2E (process 2, Fig. 16a) and then goes to the ground state (process 3, Fig. 16a) giving rise to intense NIR emission at 707 nm. The conduction electrons are captured by shallow trap level TRAP 1 (process 2, Fig. 16b) and deep trap level TRAP 2 (process 3, Fig. 16b). At the earlier stage, after stoppage of excitation, the electrons that are captured in the shallow trap are released via the conduction band (process 4, Fig. 16b) and recombine with the ionized Cr3+ ions. This dominates the initial intense persistent luminescence and gives an NIR afterglow (process 5 & 6, Fig. 16b). Then very negligible amount of electrons are released through the conduction band due to depletion of TRAP 1, most of the electrons in the deep traps (TRAP 2) directly tunnel a short distance to the nearby ionized Cr3+ ions and are captured into the energy matched Cr3+ energy levels (process 7, Fig. 16b). This tunnelling process happens at a slow rate. The electrons recombine with the Cr3+ ions and give emission (process 8, Fig. 16b). It leads to the long persistent luminescence.
 |
| | Fig. 16 (a) & (b) Persistent luminescence mechanism of Cr3+ doped MgGa2O4 phosphor (the single straight line arrows and multiple straight line arrows represent optical transition and electron transfer processes respectively). | |
4. Conclusion
In summary, Cr3+ doped and undoped MgGa2O4 nanophosphor were successfully prepared by hydrothermal method and its structural and luminescent properties were studied. The XRD pattern confirms the cubic phase and pure crystalline properties of the sample. From the FTIR spectra different bands such as Ga–O, Mg–O, N–O, C–H, –OH stretching bands were observed. From HRTEM analysis, the particle size was found to be in the range 10–70 nm. The (422), (400), (311), (111), (200) lattice planes were identified in the annealed MgGa2O4:0.25 mol% Cr3+ from the SAED pattern consistent with the peaks of the XRD pattern. The presence of Cr in the EDAX spectra confirmed the doping of Cr3+ in MgGa2O4 host. The diffuse reflectance spectra showed an absorption band near 225 nm corresponding to the band gap of the nanophosphor. The optical band gap was varied from 5.36 eV to 5.19 eV due to the incorporation of Cr3+ ion in MgGa2O4 host. Three excitation bands were observed at 225 nm, 445 nm and 558 nm from PL excitation spectrum. The PL emission spectra show the 2E(2G) → 4A2(4F) transition of Cr3+ ion at 707 nm. The phosphorescence emission spectra composed of three deconvoluted peaks at 675 nm, 707 nm and 733 nm due to spin forbidden transition 2E(2G) → 4A2(4F) of Cr3+ ion in regular octahedral environment or in distorted octahedral environment. The decay curves were fitted triple exponentially and the decay parameters were found to be decreased for the sample with lowest concentration of Cr3+ and the decay rate significantly enhanced in the annealed 0.25 mol% Cr3+ doped MgGa2O4 nanoparticles prepared by hydrothermal method than the solid state reaction prepared MgGa2O4:0.50 mol% Cr3+. Long afterglow signal was being observed for 1 hour when the sample was excited at 225 nm for 5 minutes. The small particle size and long afterglow of the MgGa2O4:Cr3+ nanoparticles in the NIR range confirm its suitability for application as a probe for in vivo bio imaging.
Acknowledgements
The author Amba Mondal gratefully acknowledges Indian School of Mines, Dhanbad for providing research fellowship funded by Government of India. The author Sourav Das and J Manam acknowledge the Department of Science and Technology, GoI for financial support for the project through grant no. SB/S2/CMP-0033/2013.
References
- Y. Jin, Y. Hu, H. Duan, L. Chen and X. Wang, RSC Adv., 2014, 4, 11360 RSC.
- W. Zeng, Y. H. Wang, S. C. Han, W. B. Chen, G. Li, Y. Z. Wang and Y. Wen, J. Mater. Chem. C, 2013, 1, 3004 RSC.
- Y. Q. Li, Y. H. Wang, Y. Gong, X. H. Xu and M. J. Zhou, Opt. Express, 2010, 18, 24853 CrossRef CAS PubMed.
- Y. H. Jin, Y. H. Hu, L. Chen, X. J. Wang, G. F. Ju and Z. F. Mou, J. Am. Ceram. Soc., 2013, 96, 3821 CrossRef CAS.
- K. Van den Eeckhout, D. Poelman and P. F. Smet, Materials, 2013, 6, 2789 CrossRef CAS.
- Z. Pan, Y. Y. Lu and F. Liu, Nat. Mater., 2012, 11, 58 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710 CrossRef CAS PubMed.
- C. H. Quek and K. W. Leong, Nanomaterials, 2012, 2, 92 CrossRef CAS.
- Z. Pan, Y. Ying Lu and F. Liu, Nat. Mater., 2012, 11, 58 CrossRef CAS PubMed.
- B. Struve and G. Huber, Appl. Phys. B, 1985, 36, 195 CrossRef.
- L. S. Forster, Chem. Rev., 1990, 90, 331 CrossRef CAS.
- H. Szymczak, M. Wardzynska and I. E. Mylnikova, J. Phys. C: Solid State Phys., 1975, 8, 3937 CrossRef CAS.
- P. I. Macfarlane, T. P. J. Han, B. Henderson and A. A. Kaminskii, Opt. Mater., 1994, 3, 15 CrossRef CAS.
- P. I. Macfarlane, T. P. J. Han, B. Henderson and A. A. Kaminskii, Inorg. Mater., 1988, 24, 579 Search PubMed.
- F. Liu, W. Yan, Y. J. Chuang, Z. Zhen, J. Xie and Z. Pan, Sci. Rep., 2013, 3, 1554 Search PubMed.
- A. Bessière, S. K. Sharma, N. Basavaraju, K. R. Priolkar, L. Binet, B. Viana, A. J. J. Bos, T. Maldiney, C. Richard, D. Scherman and D. Gourier, Chem. Mater., 2014, 26, 1365 CrossRef.
- D. Jia, L. A. Lewis and X. Wang, Electrochem. Solid-State Lett., 2010, 13, J32 CrossRef CAS.
- N. Basavaraju, S. K. Sharma, A. Bessiere, B. Viana, D. Gourierand and K. R. Priolkar, J. Phys. D: Appl. Phys., 2013, 46, 375401 CrossRef.
- A. Abdukayum, J. T. Chen, Q. Zhao and X. P. Yan, J. Am. Chem. Soc., 2013, 135, 14125 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, X. Wu, L. Hunag, D. Li, W. Fan and G. Han, J. Am. Chem. Soc., 2015, 137, 5304 CrossRef CAS PubMed.
- G. M. Whitesides, Nat. Biotechnol., 2003, 21, 1161 CrossRef CAS PubMed.
- A. D. Ostrowski, E. M. Chan, D. J. Gargas, E. M. Katz, G. Han, P. J. Schuck, D. J. Milliron and B. E. Cohen, ACS Nano, 2012, 6, 2686 CrossRef CAS PubMed.
- P. P. Paland and J. Manam, Mater. Sci. Eng. B, 2013, 178, 400 CrossRef.
- P. K. Baitha, P. P. Pal and J. Manam, Nucl. Instrum. Methods Phys. Res., Sect. A, 2014, 745, 91 CrossRef CAS.
- H. G. Haubruge, X. A. Gallez, B. Nysten and A. M. Jonas, J. Appl. Crystallogr., 2003, 36, 1019 CrossRef CAS.
- Y. Jin, Y. Hu, H. Duan, L. Chen and X. Wang, RSC Adv., 2014, 4, 11360 RSC.
- B. Rajagopal, A. V. Sarma and M. V. Ramana, Adv. Appl. Sci. Res., 2011, 2, 116 CAS.
- M. Polovka, J. Polovkova, K. Vizarova, S. Kirschnerova, L. Bielikova and M. Vrska, Vib. Spectrosc., 2006, 41, 112 CrossRef CAS.
- H. A. S. Al-Shamiri and A. S. Eid, Photonics Optoelectron., 2012, 1, 1 Search PubMed.
- J. Singh, P. K. Baitha and J. Manam, J. Rare Earths, 2015, 33, 1040 CrossRef CAS.
- V. Singh, G. Sivaramaiah, J. L. Rao, N. Singh, M. S. Pathak, H. D. Jirimali, P. K. Singh, A. K. Srivastava and S. J. Dhoble, J. Electron. Mater., 2016, 45, 1 CrossRef.
- V. Singh, R. P. S. Chakradhar, J. L. Rao and D. Kuk-Kim, J. Lumin., 2009, 129, 130 CrossRef CAS.
- A. K. Bedyal, V. Kumar, R. Prakash, O. M. Ntwaeaborwa and H. C. Swart, Appl. Surf. Sci., 2015, 329, 40 CrossRef CAS.
- M. Chowdhury and S. K. Sharma, RSC Adv., 2015, 5, 51102 RSC.
- K. P. O'Donnell and X. Chen, Appl. Phys. Lett., 1991, 58, 2925 Search PubMed.
- L. P. Sosman, R. J. M. D. Fonseca, A. D. J. Tavares, R. B. Barthem and T. Arbitta, J. Phys. Chem. Solids, 2007, 68, 22 CrossRef CAS.
- M. A. F. M. D. Silva, S. D. S. Pedro and L. P. J. Sosman, J. Alloys Compd., 2010, 492, 282 CrossRef.
- W. X. Zhang, Y. H. Wang, H. L. Li, X. S. Wang and H. Zhao, Spectrosc. Spectral Anal., 2013, 33, 31 CAS.
- D. Jia, L. A. Lewis and X. J. Wang, Electrochem. Solid-State Lett., 2010, 13, J32 CrossRef CAS.
- X. Xu, J. R. G. Chen, D. Kong, C. Gu, C. Chen and L. Kong, Opt. Mater. Express, 2013, 3, 1727 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 





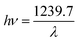 .
.