Mesoporous silica coating on hierarchical flowerlike Fe2O3 with enhanced catalytic activity for Fenton-like reaction†
Zhi-Min Cui*a,
Jing Haoa,
Jian Liub and
Wei-Guo Song*b
aKey Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry and Environment, Beihang University, Beijing 100191, PR China. E-mail: cuizhm@iccas.ac.cn
bNational Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: wsong@iccas.ac.cn
First published on 25th July 2016
Abstract
Hierarchical flowerlike Fe2O3 was coated with mesoporous silica to form a Fe2O3@mesoporous silica composite with a core/shell structure through a simple solution-based method with the assistance of Fe2O3 as a precursor. When used as a catalyst for a Fenton-like reaction for the degradation of methylene blue, the Fe2O3 mesoporous silica composite was much more active than the bare flowerlike Fe2O3, which suggests that the catalytic activity of the flowerlike Fe2O3 in the Fenton-like reaction was dramatically enhanced by the mesoporous silica coating.
The Fenton reaction based on the generation of hydroxyl radicals from the decomposition of hydrogen peroxide in the presence of ferrous ions (Fe2+) in an acidic condition is one of the most cost-effective methodologies to treat waste water.1–5 Most of the organic pollutants are degraded to low-molecular-mass carboxylic acids and CO2 in the Fenton reaction.1 Homogeneous catalysts are efficient towards the Fenton reaction; however, there are some drawbacks of homogeneously catalyzed Fenton reactions such as the need for large amounts of transition metal salts, the production of large amounts of sludge, and the need for pH value adjustments before and after Fenton reactions.3 Heterogeneous catalysts for Fenton-like reaction are attracting increasing research attention due to the advantages of allowing easier separation from the effluent and reuse without activity loss.3 In the past few years, numerous types of materials, such as clays, silicas, zeolites, metals and metal oxides, have been developed as active heterogeneous catalysts for Fenton-like reactions.6–9 Among them, iron-based materials are promising catalysts for practical application as they are low cost and storable.10,11 However, the heterogeneous Fenton-like activities of these catalysts are relatively low in normal reaction conditions without any external energy input such as light irradiation.12 Therefore, designing and developing heterogeneous catalysts with high activity and high durability are most important for Fenton-like reactions.
Recently, various strategies have been developed to improve the performance of iron-based heterogeneous Fenton-like catalysts. Reducing the size of the catalyst to the nano-scale is an effective way to increase the surface area and surface energy for a reaction.3,13,14 Loading the catalysts with small sized particles onto carriers with high surface areas can improve the dispersion of the catalysts and further improve the catalytic activity.15–17 Constructing a specific nanostructure is another way to build active Fe-based Fenton-like catalysts.18–21 Panda et al. reported a mesoporous Fe2O3–SiO2 composite catalyst that showed rapid decolourization of methyl orange by a Fenton-like reaction.22 Zheng et al. prepared an ordered mesoporous hematite by a magnesium selective leaching method and then found that the mesoporous iron oxide is an effective Fenton-like catalyst for the degradation of methylene blue (MB).12 Very recently, a yolk/shell structured Fe2O3@mesoporous silica nanoreactor was developed by our group.23 Compared with bare Fe2O3 nanoparticles, this nanoreactor exhibited enhanced catalytic activity for the Fenton-like reaction of the degradation of MB without adjusting the pH values. Liu et al. also reported Fe0@SiO2 nanoparticles with a yolk/shell structure as a nanoreactor and they demonstrated enhanced catalytic activity of the nanoreactor for the Fenton-like catalytic oxidation of phenol.24 However, developing Fe-based nanostructures with high catalytic activity and reusability for Fenton-like reactions is still desirable.
Hierarchical structured Fe2O3 is a promising catalyst for Fenton-like reactions as its nano-sized building blocks could provide a large specific surface area, more catalytically active sites and facile mass transportation pathways while their entire micro-metered size was favourable for catalyst recovery.25 However, the application of hierarchical structured Fe2O3 in Fenton-like reactions is rarely reported. In this report, a flowerlike Fe2O3 with a hierarchical structure was prepared through a simple hydrothermal method. Then, the flowerlike Fe2O3 was coated with mesoporous silica to form an Fe2O3@mesoporous silica (Fe2O3@meso-SiO2) composite with a core/shell structure. The flowerlike Fe2O3 and the Fe2O3@meso-SiO2 composite were both used as catalysts for Fenton-like reactions in the degradation of MB. It was found that the catalytic activity of the flowerlike Fe2O3 to Fenton-like reactions was dramatically enhanced by the mesoporous silica coating.
The flowerlike Fe2O3 precursor was synthesized in ethylene alcohol with a simple hydrothermal method, according to the reference.25 The X-ray diffraction (XRD) pattern of the Fe2O3 precursor (Fig. S1, ESI†) shows the emergence of diffraction peaks in striking similarity to those of other metal oxide precursors reported in the literature.26,27 The as-synthesized Fe2O3 precursor was calcined in a muffle at 500 °C to produce crystalline Fe2O3. The XRD pattern shows that the crystal structure of the obtained flowerlike Fe2O3 agrees well with α-Fe2O3 (Fig. 1a). The room-temperature hysteresis curves showed that the flowerlike Fe2O3 was weakly magnetic (Fig. S2†). Mesoporous silica was coated on the flowerlike Fe2O3 through the reaction of the Fe2O3 precursor, the base (ammonia solution), the template (cetyltrimethyl ammonium bromide, CTAB) and silica source (tetraethyl orthosilicate, TEOS) in a mixture solution of water and ethanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v).28,29 The precipitate was collected by centrifugation and then calcined in a muffle at 500 °C to produce the final Fe2O3@meso-SiO2 composite. The Fe2O3@meso-SiO2 composite has the same XRD pattern as the flowerlike Fe2O3 (Fig. 1b), indicating that the crystal structure of the flowerlike Fe2O3 was not changed after the mesoporous silica coating.
3, v/v).28,29 The precipitate was collected by centrifugation and then calcined in a muffle at 500 °C to produce the final Fe2O3@meso-SiO2 composite. The Fe2O3@meso-SiO2 composite has the same XRD pattern as the flowerlike Fe2O3 (Fig. 1b), indicating that the crystal structure of the flowerlike Fe2O3 was not changed after the mesoporous silica coating.
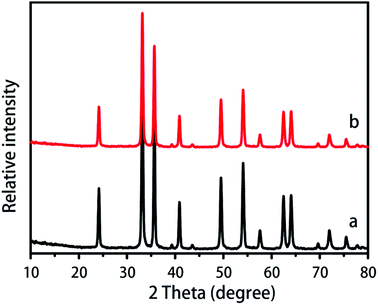 | ||
| Fig. 1 XRD pattern of the flowerlike Fe2O3 (a) and the flowerlike Fe2O3@meso-SiO2 composite (b); both the XRD patterns agree well with α-Fe2O3. | ||
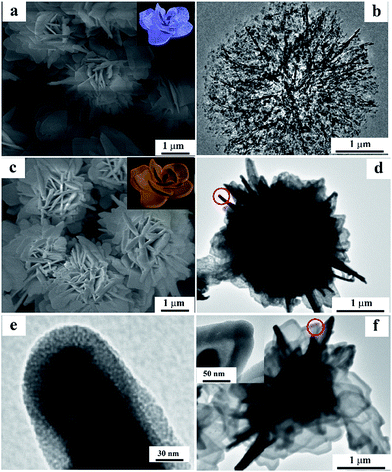
The obtained flowerlike Fe2O3 and Fe2O3@meso-SiO2 composite were further characterized by SEM and TEM. The SEM image shows that the crystalline Fe2O3 has the morphology of a flower (Fig. 2a). The TEM image shows that the flowerlike structure of Fe2O3 consists of petals as primary building units, which are composed of numerous inter-connected nanoparticles as secondary building units (Fig. 2b). After the mesoporous silica coating, the Fe2O3@meso-SiO2 composite has almost the same morphology as the flowerlike Fe2O3 except that the width of the petals of the flowers became a little thicker due to the mesoporous silica coating (Fig. 2c). The TEM image of the Fe2O3@meso-SiO2 composite further proves that the mesoporous silica coating did not change the morphology of the flowerlike Fe2O3 (Fig. 2d). The core/shell structure of the Fe2O3@meso-SiO2 composite was also demonstrated by the TEM image. The TEM image with higher magnification shows that mesoporous silica was coated uniformly on the surface of the crystalline Fe2O3. The dividing line between the Fe2O3 core and the mesoporous silica shell is visible (Fig. 3e). The thickness of the mesoporous shell is about 30 nm. The Fe2O3@meso-SiO2 composites were carefully treated with 2 M HCl to remove the metal oxide cores. Mesoporous silica hollow flowers with replica morphologies were then obtained when the metal oxide cores were removed (Fig. 2f), which suggests that the mesoporous silica coating on the flowerlike Fe2O3 had integrity and fully covered the surface of the crystalline Fe2O3. The mesoporous pores are visible in Fig. 2e and the inset of Fig. 2f, proving that the silica coating is mesoporous.
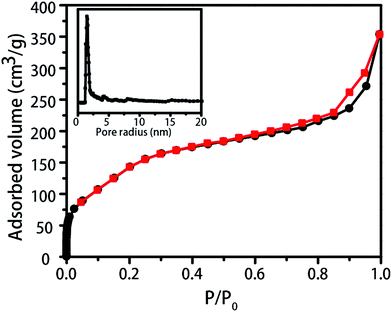 | ||
| Fig. 3 The N2 adsorption–desorption isotherms of the Fe2O3@meso-SiO2 composite; the inset is the NLDFT pore size distribution of the Fe2O3@meso-SiO2 composite. | ||
The N2 adsorption–desorption isotherms of the Fe2O3@mesoporous SiO2 composite are of type II and the hysteresis is of type H3. The remarkably sharp capillary condensation step between 0.2 and 0.4 P/P0 indicates that the sample possessed a narrow size distribution (Fig. 3).30 The pore size distribution, calculated by the Non-Local Density Functional Theory (NLDFT) method, showed that the pore size was 1.5 nm (inset of Fig. 3), which agrees well with the mesoporous pores. The mesoporous properties of the Fe2O3@meso-SiO2 composite were attributed to the mesoporous silica coating. Moreover, the Fe2O3@meso-SiO2 composite had a large BET surface area of 482.4 m2 g−1, which is much bigger than the bare flowerlike Fe2O3 (BET surface area of bare flowerlike Fe2O3 is 94.5 m2 g−1, whereas the BET surface area of mesoporous silica hollow flower is 792.4 m2 g−1). The larger BET surface area would result in enhanced adsorption ability when the Fe2O3@meso-SiO2 composite was used as a catalyst in a solution. Moreover, the sizes of the organic molecules such as methylene blue (MB, 0.7 × 0.16 nm)31 are much smaller than the size of the mesoporous pores. The mesoporous pores were big enough for the reagents to diffuse and pass through so that the reagents could arrive and react on the active sites of the flowerlike Fe2O3 core without hindrance. We envision that the Fe2O3@meso-SiO2 composite would have significantly different catalytic activity than the bare flowerlike Fe2O3 as a Fenton-like catalyst.
Fenton-like reactions were carried out for MB degradation to test the activities of the flowerlike Fe2O3 and the flowerlike Fe2O3@meso-SiO2 composites (Fig. 4). No acid or base was used to adjust the pH value of the reaction solution. At −60 min, the catalyst was added to the MB solution, and the mixture was kept in dark for 60 min to establish an adsorption–desorption equilibrium. The Fenton-like reaction was started at the 0 min mark when a definite amount of H2O2 was added to the solution. As shown in Fig. 4, the bare flowerlike Fe2O3 showed relatively low activity and less than 15% of MB was decolourized after 120 min. The catalytic activity of the flowerlike Fe2O3 is much lower than the mesoporous hematite reported in the literaure.12 The mesoporous shell itself (the inner Fe2O3 core was removed by acid etching) also showed a certain ability to decrease the MB concentration during the first 60 min and approximately 30% decrease in concentration was observed. However, the concentration of MB remained almost unchanged after the addition of H2O2, indicating that the concentration decrease was due to the adsorption of MB molecules by the mesoporous SiO2 shell.
In sharp contrast, the Fe2O3@meso-SiO2 composite was very active in these Fenton-like reactions. The Fe2O3@meso-SiO2 composite showed a similar adsorption ability to the mesoporous silica shell in the first 60 min before the addition of H2O2, but the concentration of MB decreased rapidly when the Fenton-like reaction was started by the addition of H2O2. The MB solution was fully decolourized in 120 min by the Fe2O3@meso-SiO2 composite catalyst. The extent of degradation was further analyzed by the total organic carbon (TOC) of the reaction solution. TOC analysis showed that the TOC value was reduced by 85%, relative to the original solution catalyzed by the Fe2O3@meso-SiO2 composite. Such a TOC value decrease, as well as the full MB concentration decrease, offered strong evidence for our conclusion that the MB molecules were completely degraded into water and CO2. Compared with mesoporous hematite in the literature,12 the Fe2O3@meso-SiO2 composite showed a similar catalytic activity. In addition, the reuse of the Fe2O3@meso-SiO2 composite catalyst was also tested. Although the weak magnetism of the Fe2O3@meso-SiO2 composites made them hardly available to be recovered in magnetic field (Fig. S3†), they can be easily recovered from the reaction mixture by centrifugation and reused in subsequent reaction cycles due to their micro-metered size. The reaction activity of the Fe2O3@meso-SiO2 composite to the degradation of MB was almost unchanged in the next 5 runs compared to the first cycle (Fig. S4†).
The outstanding activity of the catalyst was attributed to the specific hierarchy of the flowerlike Fe2O3 core and the mesoporous silica shell of the Fe2O3@meso-SiO2 composite. The generation of ˙OH radicals in the catalytic process was investigated according to the reaction of ˙OH radicals with terephthalic acid, producing 2-hydroxyterephthalic acid. The photoluminescence spectra intensity at 425 nm gradually increases with increasing irradiation time in the presence of different catalysts (Fig. S5†). The ˙OH radical intensity of Fe2O3@meso-SiO2 composite was notably higher than that of the bare flowerlike Fe2O3. Therefore, the degradation activity of the Fe2O3@meso-SiO2 composite could be expected to be higher than that of bare flowerlike Fe2O3. In addition, the Fenton-like catalytic reaction mainly occurs on the catalyst surface, near a neutral environment.12 Therefore, the adsorption of reagents onto the catalyst plays an important role in this heterogeneous Fenton-like reaction. In case of the flowerlike Fe2O3, the nano-sized building blocks of the flowerlike Fe2O3 provide a large surface area for contact and sufficient active sites for the decomposition of H2O2 to produce ˙OH radicals in the Fenton-like reaction. The easy escape of the ˙OH radicals and low mass transfer of MB molecules to the surface of the catalyst limited the activity of the bare flowerlike Fe2O3. In the Fe2O3@meso-SiO2 composite, the mesoporous shell of the Fe2O3@meso-SiO2 composite can adsorb the MB molecules from the bulk solution and enrich them on the surface of the flowerlike Fe2O3 core, whereas ˙OH radicals generated on the surface of the flowerlike Fe2O3 core were also trapped by the mesoporous silica shell, resulting in a higher reactant concentration on the surface of the flowerlike Fe2O3. The degrading reactions were confined within the mesoporous pores, where the Fe2O3 core was more accessible than for the bare flowerlike Fe2O3 in the bulk solution. Moreover, contact opportunities between MB molecules and hydroxyl radicals were also increased in the Fe2O3@meso-SiO2 composite compared with the bare flowerlike Fe2O3 due to the mesoporous silica coating. Thus, the catalytic activity of the flowerlike Fe2O3 was enhanced by the mesoporous silica coating.
Conclusions
To summarize, a flowerlike Fe2O3 with hierarchical structure was synthesized through a simple hydrothermal method. The flowerlike Fe2O3 was coated with mesoporous silica to form an Fe2O3@meso-SiO2 composite in aqueous media. SEM and TEM images demonstrated that in the Fe2O3@meso-SiO2 composite, mesoporous silica was coated uniformly and completely on the surface of the flowerlike Fe2O3 core. When used as a catalyst for the Fenton-like reaction in the degradation of MB, the Fe2O3@meso-SiO2 composite is much more active than the bare flowerlike Fe2O3, which suggests that the mesoporous silica coating can enhance the catalytic activity of the hierarchical Fe2O3.Acknowledgements
We are thankful for the financial support from the National Basic Research Program of China (MOST 2013CB933004, 2014CB931802), the National Natural Science Foundation of China (NSFC 51302008, 51502089), the Fundamental Research Funds for the Central Universities (2016MS03) and the Chinese Academy of Sciences.Notes and references
- M. Munoz, Z. M. de Pedro, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2015, 176, 249–265 CrossRef.
- M. Umar, H. A. Aziz and M. S. Yusoff, Waste Manag., 2010, 30, 2113–2121 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, S. Navalon, M. Alvaro and H. Garcia, ChemSusChem, 2012, 5, 46–64 CrossRef CAS PubMed.
- K. Ikehata and M. G. El-Din, J. Environ. Eng. Sci., 2006, 5, 81–135 CrossRef CAS.
- Y. J. Wang, G. H. Zhao, S. N. Chai, H. Y. Zhao and Y. B. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 842–852 CAS.
- S. Navalon, M. Alvaro and H. Garcia, Appl. Catal., B, 2010, 99, 1–26 CrossRef CAS.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro and H. Garcia, ChemSusChem, 2011, 4, 1712–1730 CrossRef CAS PubMed.
- J. Qu, Y. Yu, C. Y. Cao and W. G. Song, Chem.–Eur. J., 2013, 19, 11172–11177 CrossRef CAS PubMed.
- K. A. Sashkina, V. S. Labko, N. A. Rudina, V. N. Parmon and E. V. Parkhomchuk, J. Catal., 2013, 299, 44–52 CrossRef CAS.
- M. C. Pereira, L. C. A. Oliveira and E. Murad, Clay Miner., 2012, 47, 285–302 CrossRef CAS.
- S. R. Pouran, A. A. A. Raman and W. Daud, J. Cleaner Prod., 2014, 64, 24–35 CrossRef.
- C. M. Zheng, X. Z. Cheng, P. P. Chen, C. W. Yang, S. M. Bao, J. Xia, M. L. Guo and X. H. Sun, RSC Adv., 2015, 5, 40872–40883 RSC.
- M. Li, Q. Gao, T. Wang, Y. S. Gong, B. Han, K. S. Xia and C. G. Zhou, Mater. Des., 2016, 97, 341–348 CrossRef CAS.
- X. G. Shi, A. Tian, J. H. You, Z. Q. Yu, H. Yang and X. X. Xue, Mater. Lett., 2016, 169, 153–156 CrossRef CAS.
- X. F. Li, X. Liu, L. L. Xu, Y. Z. Wen, J. Q. Ma and Z. C. Wu, Appl. Catal., B, 2015, 165, 79–86 CrossRef CAS.
- W. L. Guo, F. F. Hao, X. X. Yue, Z. H. Liu, Q. Y. Zhang, X. H. Li and J. Wei, Environ. Prog. Sustainable Energy, 2016, 35, 48–55 CrossRef CAS.
- H. L. Li, J. J. Zhu, P. Xiao, Y. Y. Zhan, K. L. Lv, L. Y. Wu and M. Li, Microporous Mesoporous Mater., 2016, 221, 159–166 CrossRef CAS.
- X. Li, F. Y. Gai, B. Y. Guan, Y. Zhang, Y. L. Liu and Q. S. Huo, J. Mater. Chem. A, 2015, 3, 3988–3994 CAS.
- T. Zeng, X. L. Zhang, S. H. Wang, Y. R. Ma, H. Y. Niu and Y. Q. Cai, Chem.–Eur. J., 2014, 20, 6474–6481 CrossRef CAS PubMed.
- J. Wang, C. Liu, L. Tong, J. S. Li, R. Luo, J. W. Qi, Y. Li and L. J. Wang, RSC Adv., 2015, 5, 69593–69605 RSC.
- P. H. Shao, J. Y. Tian, B. R. Liu, W. X. Shi, S. S. Gao, Y. L. Song, M. Ling and F. Y. Cui, Nanoscale, 2015, 7, 14254–14263 RSC.
- N. Panda, H. Sahoo and S. Mohapatra, J. Hazard. Mater., 2011, 185, 359–365 CrossRef CAS PubMed.
- Z. M. Cui, Z. Chen, C. Y. Cao, L. Jiang and W. G. Song, Chem. Commun., 2013, 49, 2332–2334 RSC.
- C. Liu, J. S. Li, J. W. Qi, J. Wang, R. Luo, J. Y. Shen, X. Y. Sun, W. Q. Han and L. J. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 13167–13173 CAS.
- J.-S. Hu, L.-S. Zhong, W.-G. Song and L.-J. Wan, Adv. Mater., 2008, 20, 2977–2982 CrossRef CAS.
- L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426–2431 CrossRef CAS.
- A. M. Cao, J. S. Hu, H. P. Liang and L. J. Wan, Angew. Chem., Int. Ed., 2005, 44, 4391–4395 CrossRef CAS PubMed.
- Z. Chen, Z. M. Cui, F. Niu, L. Jiang and W. G. Song, Chem. Commun., 2010, 46, 6524–6526 RSC.
- Z. M. Cui, Z. Chen, C. Y. Cao, W. G. Song and L. Jiang, Chem. Commun., 2013, 49, 6093–6095 RSC.
- Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466–8473 CrossRef CAS PubMed.
- H. Awala, E. Leite, L. Saint-Marcel, G. Clet, R. Retoux, I. Naydenova and S. Mintova, New J. Chem., 2016, 40, 4277–4284 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experiment. See DOI: 10.1039/c6ra15092f |
| This journal is © The Royal Society of Chemistry 2016 |