Evaluation of a drinking water treatment process involving directly recycling filter backwash water using physico-chemical analysis and toxicity assay†
Abstract
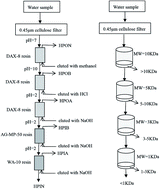
Recycling the filter backwash water of a drinking water treatment plant (DWTP) was considered as a feasible method to enhance the efficiencies of pollutant removal and water conservation. In this study, the purification efficiency and water quality evaluation were investigated in a DWTP with a direct recycling of sand filters backwash water (SFBW) at recycling rates of 0–30%. The concentrations of dissolved organic carbon and dissolved nitrogen matters, and the formation potentials of carbonated or nitrogenous disinfection by-products did not obviously increase or were even lower than that without recycling when the recycling rate of SFBW was less than 20%. The di-haloacetamides (DCAcAm) accounted for the majority of HAcAms formed during chlorination in all water extracts, followed by tri-HAcAms and, to a much lower extent, mono-HAcAms. The immobilization of Daphnia magna (D. magna) increased with the increasing exposure concentration of water extracts during a 48 h period. The immobilization of D. magna exposed to water treated by recycling SFBW at a recycling rate below 20% was nearly equivalent to that without a recycling process. When the recycling rate was more than 20%, there was a significant increase in the superoxide dismutase (SOD) activity of D. magna, whereas a significant inhibition observed in the catalase (CAT) activity, which reflected a damaged defense chain caused by the toxic substances including chlorinated and nitrogenous disinfection by-products such as DCAcAm. When the recycling rate was more than 20%, there was a statistically significant difference (p > 0.05) between the recycling rate of SFBW and the toxicity effect of water sample extracts during the recycling trial. It was also identified that the main precursors of DCAcAm were hydrophilic and low molecular weight fractions of dissolved organic nitrogen.

 Please wait while we load your content...
Please wait while we load your content...