DOI:
10.1039/C6RA14903K
(Paper)
RSC Adv., 2016,
6, 79853-79858
Removal of methylene blue from aqueous solution using porous starch-g-poly(acrylic acid) superadsorbents
Received
8th June 2016
, Accepted 16th August 2016
First published on 16th August 2016
Abstract
Porous starch-g-poly(acrylic acid) (St-g-PAA) superadsorbents have been prepared by free radical graft polymerization of AA onto potato starch in aqueous solutions in the presence of Triton X-100. The porous St-g-PAA superadsorbent was characterized by scanning electron microscopy. Batch adsorption experiments were carried out for the removal of methylene blue (MB) from aqueous solutions using the St-g-PAA superadsorbents. The effects of the pH of MB solutions and the weight ratio of AA to starch on the adsorption capacity were investigated. In addition, the adsorption kinetics and adsorption isotherms were analyzed by studying the effects of the adsorption time and the initial MB concentration on the adsorption capacity. The results indicates that the St-g-PAA superadsorbent prepared with an AA/starch weight ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a porous structure and has the highest adsorption capacity for MB (1532 mg g−1). In addition, the adsorption capacity increases with increasing pH and MB concentration of the MB solutions. The adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption behaviors of the St-g-PAA superadsorbent for MB indicate that the adsorption kinetics and isotherms are in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively.
1 has a porous structure and has the highest adsorption capacity for MB (1532 mg g−1). In addition, the adsorption capacity increases with increasing pH and MB concentration of the MB solutions. The adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption behaviors of the St-g-PAA superadsorbent for MB indicate that the adsorption kinetics and isotherms are in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively.
1. Introduction
Colored wastewater containing organic dyes is produced in many fields including textiles, paper and plastics.1,2 Because of long-term accumulation, in some areas the concentration of organic dyes in wastewater is very high. The dye-containing wastewater can mix with surface water and groundwater, and finally transfer to drinking water. Organic dyes in wastewater can reduce light penetration of water bodies and increase the chemical oxygen demand. Moreover, the dye-containing wastewater can cause serious side-effects on human and animals.3 For example, the most commonly used dye, methylene blue (MB), could cause permanent injury to eyes.4 Thus, effective removal of organic dyes from wastewater is an urgent issue and has recently attracted great attention.5
For the removal of organic dyes from wastewater, many methods have been used, e.g., adsorption, coagulation and flocculation and membrane separation, etc.6,7 However, the effective removal of organic dyes is still very challenging.8,9 Different from the other methods, the adsorption method has advantages in design and application.7,10 Also, the adsorbents can be easily regenerated.
Various kinds of materials including activated carbon, clays, functional polymers, industrial wastes and by-products have been used as adsorbents for the removal of organic dyes from wastewater. However, adsorbents with high adsorption capacity for organic dyes are rare. The low adsorption capacity of most adsorbents hindered their applications because the adsorbents have to be frequently regenerated and/or replaced. These adsorbents cannot be used for removal of dyes in high concentration. Also, some adsorbents like activated carbon are expensive for wide practical applications. With the development of novel adsorbents for the removal of organic dyes from water, it has been gradually recognized that preparation of novel low-cost adsorbents with high adsorption capacity is an effective approach for water decontamination.11
Here, we report application of the porous starch-g-poly(acrylic acid) (St-g-PAA) superadsorbents for the removal of MB, the model organic cationic dye (Scheme 1a), from water. Starch is one of the most abundant and inexpensive biopolymers.12 Adsorbents based polysaccharides are attracting much attention due to their biocompatibility and biodegradability. Polysaccharides such as starch, chitosan and cellulose have been used for preparing adsorbents.13,14 The morphology of the superadsorbent was characterized by scanning electron microscopy (SEM). The effects of the weight ratio of AA to starch and the pH of MB solutions on adsorption capacity of the St-g-PAA superadsorbent were studied. The adsorption kinetics and adsorption isotherms were also analyzed. The St-g-PAA superadsorbent showed high adsorption capacity and fast adsorption for MB.
 |
| | Scheme 1 (a) Structure of MB and (b) schematic illustration of the St-g-PAA superadsorbents. | |
2. Experimental
2.1 Materials
Potato starch was supplied by Weifang Fushan Starch Co., Ltd (Weifang, China). AA (chemically pure) was distilled under reduced pressure before use. AA, ammonium persulfate (analytical grade), N,N′-methylenebisacrylamide (analytical grade) and MB (analytical grade) were purchased from Shanghai Chemical Reagent Corp. (Shanghai, China). Ammonium persulfate was recrystallized from water before use. Triton X-100 was purchased from Aike Reagent Corp. (Chengdu, China). Other agents used were all analytical grade.
2.2 Preparation of porous St-g-PAA superadsorbents
First of all, certain amount of potato starch was dispersed in 42.0 mL of distilled water in a flask (250 mL) with three-necks. The dispersion was vigorously stirred and gelatinized at 95 °C for 60 min under nitrogen atmosphere, and then cooled to 50 °C. Then, 0.20 g of Triton X-100 was added into the gelatinized starch solution and stirred vigorously for 30 min to form the foamed starch. A mixture of 7.2 g of AA, 21 mL of NaOH solution (3.0 M), 60.0 mg of N,N′-methylenebisacrylamide and 180 mg of ammonium persulfate was quickly charged into the above foamed starch. The temperature was kept at 65 °C for 3 h. The product was washed with distilled water, and then dewatered in 100 mL of ethanol for 24 h, and then dried in a vacuum oven at 50 °C. The samples with different weight ratio of AA to starch (mAA/mstarch) in the range 3 to 8 were prepared. The samples were crushed with a mortar and all samples used for test had a particle size in the range of 60–80 mesh.
2.3 Adsorption of MB
The adsorption experiments were carried out in a batch process using MB aqueous solutions.7 50 mg of St-g-PAA was added into 100 mL of the MB solution (800–1800 mg L−1) in a 250 mL conical flask. The mixture was agitated in a thermostatic mechanical shaker for 2 h at 25 °C to ensure that adsorption equilibrium was achieved. The mixture was centrifuged using a high speed tabletop refrigerated centrifuge and the residual concentration of MB remained in the solution was determined using a UV-Vis spectrophotometer (UV2400, Niuyin Huaxin Technology Co., Ltd, Beijing, China) at 665 nm according to the calibration curve. The adsorption capacity of the samples for MB was calculated according to the following equation.where qe is the adsorption capacity of the sample for MB at equilibrium (mg g−1), C0 is the initial concentration of MB in solution (mg L−1), Ce is the equilibrium concentration of MB in solution (mg L−1), m is the mass of the sample used (g) and V is the volume of the MB solution (L). The adsorption experiments were carried three times and the average data were presented.
2.4 Characterization
The surface microstructure of the representative superadsorbent was observed via SEM (JSM-6510A, JEOL, Ltd, Japan). The sample was fixed on a copper stub and coated with gold before SEM observation. The MB concentrations in solutions were determined using a UV-Vis spectrophotometer (UV2400, Niuyin Huaxin Technology Co., Ltd, Beijing, China) at 665 nm according to the calibration curve. The specific surface area of the sample was determined by the Brunauer–Emmett–Teller (BET) method (ASAP 2020 M, Micromeritics Instrument Corporation, USA).
3. Results and discussion
3.1 Structure and surface morphology of porous St-g-PAA superadsorbents
The St-g-PAA superadsorbents were prepared by free radical graft polymerization of AA onto starch using N,N′-methylenebisacrylamide as the crosslinker and ammonium persulfate as the initiator. The schematic illustration of the St-g-PAA superadsorbents is shown in Scheme 1b. The free radical graft polymerization between starch and AA has formed a crosslinked network. A lot of pores can be seen on the surface of the sample as shown in Fig. 1. The pore diameter of the St-g-PAA superadsorbent is not uniform. A lot of micropores and nanopores can be seen via SEM. The specific surface area of the sample is 45.1 m2 g−1. Triton X-100 is responsible for the high porosity and specific surface area of the St-g-PAA superadsorbent according to our previous report.15 As is well known, a porous surface is important for the adsorption of organic dyes from water.
 |
| | Fig. 1 SEM image of the porous St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | |
3.2 Effect of pH on adsorption capacity
It was reported that the pH of the dye-containing solution has great influence on the adsorption capacity of an adsorbent. So, the effect of pH of the MB aqueous solution on adsorption of MB by the St-g-PAA superadsorbent was studied and the results are shown in Fig. 2. The adsorption capacity for MB increases evidently from 872 to 1523 mg g−1 with increasing the pH from 2.0 to 6.0. The further increase in the pH has little influence on the adsorption capacity, which remains around 1500 mg g−1. The increase of the adsorption capacity for MB with pH is because of the following facts. At higher pH, there are more –COO− groups on the network of the St-g-PAA superadsorbent, which could interact with the MB molecules. Thus, an increase of the absorption capacity for MB was observed with the increase of pH. In addition, the electrostatic repulsion among the adjacent –COO− groups induces expansion of the crosslinked network of the superadsorbent, which is also responsible for the increase of the absorption capacity at higher pH. Similar result has been reported for the adsorption of MB by the chitosan-g-PAA/montmorillonite superadsorbent nanocomposite7 and the superadsorbent based on gum arabic, PAA and polyacrylamide.16 The adsorption mechanism of MB using PAA-based hydrogels is well known.7,17 We think the adsorption of MB by the St-g-PAA superadsorbents should follow the same mechanism.
 |
| | Fig. 2 Variation of adsorption capacity of the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB with pH of the MB solution (1400 mg L−1). 1) for MB with pH of the MB solution (1400 mg L−1). | |
3.3 Effect of mAA/mstarch on adsorption capacity
The variation of adsorption capacity of the St-g-PAA superadsorbents for MB with mAA/mstarch is shown in Fig. 3. It is clear that the adsorption capacity increases from 1272 to 1523 mg g−1 with increasing the mAA/mstarch from 3 to 6. With a mAA/mstarch of 6, the superadsorbent has the highest adsorption of 1523 mg g−1. The superadsorbent contains more PAA chains with increasing the mAA/mstarch, which increases the content of –COO− groups on the network of the St-g-PAA superadsorbent, and then enhances the adsorption capacity. Also, more Na+ ions are generated in the polymeric network owing to neutralization of the grafted PAA in the superadsorbent. Consequently, the osmotic pressure difference between the polymeric network and the external MB solution increased, which also results in the higher adsorption capacity of the superadsorbent for MB. However, the adsorption capacity slightly decreases with further increasing the mAA/mstarch to 8. This is owing to the fact that too many PAA chains tend to form water-soluble materials, which is unfavorable for the adsorption of MB.18
 |
| | Fig. 3 Variation of adsorption capacity of the St-g-PAA superadsorbent for MB (1400 mg L−1, pH 6.0) with mAA/mstarch. | |
3.4 Adsorption kinetics
The effect of contact time on the adsorption capacity of the St-g-PAA superadsorbent for MB is shown in Fig. 4. The adsorption capacity increases quickly with the contact time in the first 20 min, and then increases slowly with time until the adsorption equilibrium is achieved in 30 min. This is faster than many of the previously reported adsorbents for MB (Table 1).7,19–25 This is in accordance with the SEM observation of the porous surface microstructure. The porous structure makes the penetration of MB solution easier into the 3D polymeric network. The fast adsorption of adsorbents for pollutants from water is very important for their practical applications.
 |
| | Fig. 4 Effect of the adsorption time on adsorption capacity of the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB (1400 mg L−1, pH 6.0). 1) for MB (1400 mg L−1, pH 6.0). | |
Table 1 Time required to adsorption equilibrium for the previously reported hydrogels and the St-g-PAA superadsorbent prepared in this study
| Samples |
Equilibrium time/h |
Ref. |
| Chitosan-g-poly(acrylic acid)/montmorillonite hydrogel |
1 |
7 |
| Poly(DMAA-co-AMPSNa)/clay hydrogel |
50 |
19 |
| NaAlg-g-poly(acrylic acid-co-acryl amide)/clinoptilolite hydrogel |
24 |
20 |
| Hemicellulose-based hydrogel |
59 |
21 |
| Starch immobilized humic acid hydrogel |
1.3 |
22 |
| Gum karaya-g-poly(acrylic acid-acrylamide)/SiO2 hydrogel |
1.25 |
23 |
| Polyaniline hydrogel |
11 |
24 |
| Graphene oxide based hydrogel |
0.75 |
25 |
| This study |
0.5 |
— |
To investigate the adsorption mechanism of the St-g-PAA superadsorbent for MB in aqueous solutions, the adsorption kinetics was analyzed. The pseudo-first-order kinetic model is valid only for the initial adsorption period as reported in a previous literature.26 Thus, the pseudo-second order kinetic model was used to evaluate the experimental data. The pseudo-second order kinetics model is shown according to eqn (2).27
where
qe and
qt (mg g
−1) are the adsorption capacities at equilibrium and at time
t, respectively.
k (g mg
−1 min
−1) is the rate constant of the pseudo-second order adsorption.
The adsorption kinetics curve of MB onto the St-g-PAA superadsorbent fitted to the pseudo-second order model is shown in Fig. 5. It can be seen that the experimental data fit very well with the pseudo-second order model. The R2 value for the pseudo-second order kinetic model is 0.9971. Moreover, the qe calculated using the pseudo-second order kinetic model is 1666 mg g−1, which is comparable with the experimental result of 1532 mg g−1. The results indicate that the adsorption process of the porous St-g-PAA superadsorbent for MB can be well simulated by the pseudo-second order kinetic model.
 |
| | Fig. 5 Pseudo-second-order model for the adsorption of MB (1400 mg L−1, pH 6.0) onto the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | |
3.5 Adsorption isotherms
The influence of the MB concentration on the equilibrium adsorption capacity of the St-g-PAA superadsorbent is shown in Fig. 6. The results indicate that the initial MB concentration has great influence on the equilibrium adsorption capacity of the St-g-PAA superadsorbent. The adsorption capacity increases evidently from 1320 to 1532 mg g−1 with increasing the MB concentration from 800 to 1400 mg L−1. The equilibrium adsorption capacity remains almost constant with further increase of the initial MB concentration to 1800 mg L−1. This is because the aggregation of MB molecules at high concentration makes it impossible for the MB molecules to diffuse deeper into the network of the St-g-PAA superadsorbent.7
 |
| | Fig. 6 Effect of the initial MB concentration on equilibrium adsorption capacity of the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB (pH 6.0). 1) for MB (pH 6.0). | |
Adsorption isotherms are important for the description of how the adsorbate interacts with the adsorbent. So, the correlation of equilibrium data using a theoretical equation is essential for the interpretation of the adsorption mechanism.28,29 The interaction between adsorbate and adsorbent are frequently interpreted using the Langmuir and the Freundlich isotherm models. The Langmuir isotherm model and the Freundlich isotherm model are shown by eqn (3) and (4), respectively.30,31
| | |
Ce/qe = 1/qmaxb + Ce/qmax
| (3) |
| |
log(qe) = log(Ce)/n + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K
| (4) |
where
qe is the amount of MB adsorbed at equilibrium (mg g
−1),
Ce is the liquid-phase MB concentration at equilibrium (mg L
−1),
qmax is the maximum adsorption capacity of the adsorbent (mg g
−1), and
b is the Langmuir adsorption constant (L mg
−1).
K is the Freundlich isotherm constant (L g
−1) and 1/
n is the heterogeneity factor.
Two adsorption isotherms were constructed by plotting Ce/qe versus Ce (Langmuir isotherm model) and log(qe) versus log(Ce) (Freundlich isotherm model). The adsorption of MB onto St-g-PAA fitted to the Langmuir and Freundlich isotherm models are shown in Fig. 7 and 8, respectively. As can be seen, the experimental data fit very well with the Langmuir isotherm model. The Langmuir plot and the trend line are almost entirely coincident with each other. However, the experimental data do not fit very well with the Freundlich isotherm model and there is a great difference between the Freundlich plot and the trend line. It can be seen that the linear coefficient (R2) for the Langmuir isotherm model is 0.9968. However, the R2 for the Freundlich isotherm model is 0.9387, which indicates that this model does not describe very well the adsorption process of the St-g-PAA superadsorbent for MB. In addition, the qmax for the adsorption of MB onto the St-g-PAA superadsorbent calculated from the Langmuir model is close to the experimental data. Thus, compared to the Freundlich model, the Langmuir model is much better to describe the adsorption of MB onto the St-g-PAA superadsorbent. This means the monolayer coverage of MB has formed on the surface of the St-g-PAA superadsorbent.
 |
| | Fig. 7 Langmuir plot for the adsorption of MB (pH 6.0) by the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | |
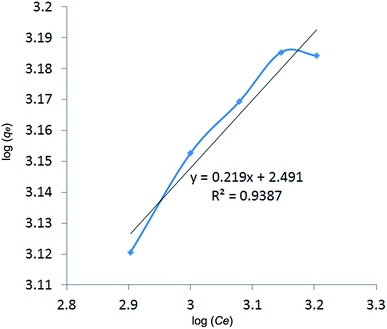 |
| | Fig. 8 Freundlich plot for the adsorption of MB (pH 6.0) by the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | |
3.6 Recycle of St-g-PAA superadsorbents
To evaluate the effect of recycle on the adsorption capacity, the consecutive adsorption–desorption process was performed five times by using the 0.05 M HCl aqueous solution as the eluent. The adsorption capacity decreases gradually with the increase of the reuse times as shown in Fig. 9. HCl solution was used as the eluent during the desorption process. Thus, the –COO− groups of the superadsorbents were changed to –COOH, which should be responsible for the decrease of the adsorption capacity. In addition, a part of adsorption sites may bind stably with MB and cannot be desorbed. The increase of the reuse time resulted in a decrease of the adsorption capacity, but a reasonable adsorption capacity was retained. The adsorption capacity was higher than 1250 mg g−1 after used for five times, which means that the St-g-PAA superadsorbent is a highly recyclable adsorbent.
 |
| | Fig. 9 Reusability of the St-g-PAA superadsorbent (mAA/mstarch = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for adsorption of MB. 1) for adsorption of MB. | |
4. Conclusions
Porous St-g-PAA superadsorbents were prepared by free radical graft polymerization between AA and potato starch in the aqueous phase by using Triton X-100 as the pore forming agent. The St-g-PAA superadsorbent prepared with an AA/starch weight ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a porous structure and has the highest adsorption capacity of 1532 mg g−1 for MB. In addition, the pH and the MB concentration of the MB aqueous solutions have great influences on the adsorption capacity. It is interesting that the adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption kinetics and isotherms were in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively. We believe that the porous St-g-PAA superadsorbents may find applications in removal of cationic organic dyes from wastewater owing to their very high adsorption capacity and adsorption rate.
1 has a porous structure and has the highest adsorption capacity of 1532 mg g−1 for MB. In addition, the pH and the MB concentration of the MB aqueous solutions have great influences on the adsorption capacity. It is interesting that the adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption kinetics and isotherms were in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively. We believe that the porous St-g-PAA superadsorbents may find applications in removal of cationic organic dyes from wastewater owing to their very high adsorption capacity and adsorption rate.
Acknowledgements
We are grateful for financial support of the Weifang University of Science and Technology.
Notes and references
- M. S. Chiou, P. Y. Ho and H. Y. Li, Dyes and Pigments, 2004, 60, 69–84 CrossRef CAS.
- E. Karadag, D. Saraydm and O. Güven, Polym. Bull., 1996, 36, 745–752 CAS.
- N. Akhtar, J. Iqbal and M. Iqbal, Eng. Life Sci., 2004, 4, 171–178 CrossRef CAS.
- M. Rafatullaha, O. R. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef PubMed.
- Q. X. Zhou and M. E. Wang, J. Soils Sediments, 2010, 10, 1324–1334 CrossRef CAS.
- J. Tang, B. Mu, W. B. Wang, M. S. Zheng and A. Q. Wang, RSC Adv., 2016, 6, 36534–36543 RSC.
- L. Wang, J. P. Zhang and A. Q. Wang, Colloids Surf., A, 2008, 322, 47–53 CrossRef CAS.
- M. A. Salem, R. G. Elsharkawy and M. F. Hablas, Eur. Polym. J., 2016, 75, 577–590 CrossRef CAS.
- C. L. Zhang, L. Zhu, W. Li and X. Zheng, J. Appl. Polym. Sci., 2016, 133, 43448 Search PubMed.
- J. P. Zhang and A. Q. Wang, J. Chem. Eng. Data, 2010, 55, 2379–2384 CrossRef CAS.
- J. P. Zhang, L. Wu, Y. J. Zhang and A. Q. Wang, J. Mater. Chem. A, 2015, 3, 18475–18482 CAS.
- M. E. Karlsson, A. M. Leeman, I. M. E. Bjöck and A. C. Eliasson, Food Chem., 2007, 100, 136–146 CrossRef CAS.
- J. Berger, M. Reist, A. Chenite, O. Felt-Baeyens, J. M. Mayer and R. Gurny, Int. J. Pharm., 2005, 288, 17–25 CrossRef CAS PubMed.
- S. P. Bhuniya, S. Rahma, A. J. Satyanand, M. M. Gharia and A. M. Dave, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1650–1658 CrossRef CAS.
- Q. Y. Wei, Iran. Polym. J., 2014, 23, 637–643 CrossRef CAS.
- A. T. Paulino, M. R. Guilherme, A. V. Reis, G. M. Campese, E. C. Muniz and J. Nozaki, J. Colloid Interface Sci., 2006, 301, 55–62 CrossRef CAS PubMed.
- Y. Z. Wang, X. N. Shi, W. B. Wang and A. Q. Wang, J. Appl. Polym. Sci., 2013, 129, 1080–1088 CrossRef CAS.
- Z. Reyes, C. F. Clark, M. Comas, C. R. Russell and C. E. Rist, Nucl. Technol., 1969, 6, 509–517 CAS.
- S. Bao, D. B. Wu, Q. G. Wang and T. Su, PLoS One, 2014, 9, e88802 Search PubMed.
- R. Azam, O. Ali and S. Dariush, Fibers Polym., 2015, 16, 354–362 CrossRef.
- X. F. Sun, Z. Gan, Z. X. Jing, H. H. Wang, D. Wang and Y. N. Jin, J. Appl. Polym. Sci., 2015, 132, 41606 Search PubMed.
- R. P. Chen, Y. L. Zhang, L. F. Shen, X. Y. Wang, J. Q. Chen, A. J. Ma and W. M. Jiang, Chem. Eng. J., 2015, 268, 348–355 CrossRef CAS.
- H. Mittal, A. Maity and S. S. Ray, Chem. Eng. J., 2015, 279, 166–179 CrossRef CAS.
- B. Yan, Z. H. Chen, L. Cai, Z. M. Chen, J. W. Fu and Q. Xu, Appl. Surf. Sci., 2015, 356, 39–47 CrossRef CAS.
- A. Pourjavadi, M. Nazari, B. Kabiri, S. H. Hosseinia and C. Bennett, RSC Adv., 2016, 6, 10430–10437 RSC.
- V. C. Srivastava, M. M. Swamy, I. D. Mall, B. Prasad and I. M. Mishra, Colloids Surf., A, 2006, 272, 89–104 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- J. P. Zhang, Y. L. Jin and A. Q. Wang, Environ. Technol., 2011, 32, 523–531 CrossRef CAS PubMed.
- G. Y. Tian, W. B. Wang, Y. R. Kang and A. Q. Wang, J. Environ. Sci., 2016, 41, 33–43 CrossRef PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. M. F. Freundlich, Z. Phys. Chem., 1906, 57, 385–470 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a porous structure and has the highest adsorption capacity for MB (1532 mg g−1). In addition, the adsorption capacity increases with increasing pH and MB concentration of the MB solutions. The adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption behaviors of the St-g-PAA superadsorbent for MB indicate that the adsorption kinetics and isotherms are in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively.
1 has a porous structure and has the highest adsorption capacity for MB (1532 mg g−1). In addition, the adsorption capacity increases with increasing pH and MB concentration of the MB solutions. The adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption behaviors of the St-g-PAA superadsorbent for MB indicate that the adsorption kinetics and isotherms are in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB with pH of the MB solution (1400 mg L−1).
1) for MB with pH of the MB solution (1400 mg L−1).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB (1400 mg L−1, pH 6.0).
1) for MB (1400 mg L−1, pH 6.0).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for MB (pH 6.0).
1) for MB (pH 6.0).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has a porous structure and has the highest adsorption capacity of 1532 mg g−1 for MB. In addition, the pH and the MB concentration of the MB aqueous solutions have great influences on the adsorption capacity. It is interesting that the adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption kinetics and isotherms were in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively. We believe that the porous St-g-PAA superadsorbents may find applications in removal of cationic organic dyes from wastewater owing to their very high adsorption capacity and adsorption rate.
1 has a porous structure and has the highest adsorption capacity of 1532 mg g−1 for MB. In addition, the pH and the MB concentration of the MB aqueous solutions have great influences on the adsorption capacity. It is interesting that the adsorption equilibrium can be achieved in 30 min, which is faster than many of the previously reported adsorbents for MB. The adsorption kinetics and isotherms were in good agreement with the pseudo-second-order equation and the Langmuir equation, respectively. We believe that the porous St-g-PAA superadsorbents may find applications in removal of cationic organic dyes from wastewater owing to their very high adsorption capacity and adsorption rate.



