High photocatalytic activity of plasmonic Ag@AgCl/Zn2SnO4 nanocomposites synthesized using hydrothermal method†
Monaam Ben Ali*ab,
Abderrahmane Hamdia,
Habib Elhouichetab,
Brigitte Sieberc,
Ahmed Addadc,
Yannick Coffinierd,
Luc Boussekeye,
Mokhtar Férida,
Sabine Szuneritsd and
Rabah Boukherroub*d
aLaboratoire de Physico-Chimie des Matériaux Minéraux et leurs Applications, Centre National de Recherches en Sciences des Matériaux, B.P. 95 Hammam-Lif, 2050, Tunisia. E-mail: monaambenali@yahoo.fr
bDépartement de Physique, Faculté des Sciences de Tunis, Université Tunis-El Manar, 2092, Tunisia
cUMET, UMR CNRS 8207, Université Lille 1, 59655 Villeneuve d'Ascq Cédex, France
dInstitut d’Electronique, de Microélectronique et de Nanotechnologie (IEMN), UMR CNRS 8520, Université Lille 1, Avenue Poincaré – BP60069, 59652 Villeneuve d'Ascq, France. E-mail: rabah.boukherroub@iemn.univ-lille1.fr
eLASIR, UMR CNRS 8516, Université Lille 1, 59655 Villeneuve d'Ascq Cédex, France
First published on 18th August 2016
Abstract
Ag@AgCl/Zn2SnO4 (ZTO) nanocomposites were successfully prepared by a hydrothermal method. The morphology, structure, composition, and optical properties of the developed composites were examined using scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy dispersive X-ray (EDX) spectroscopy, UV-visible (UV-vis) spectrophotometry, X-ray photoelectron spectroscopy (XPS), and photoluminescence techniques. All analysis confirmed the anchoring of Ag@AgCl on ZTO. The photocatalytic activity of the Ag@AgCl/ZTO nanocomposites was evaluated for the photodegradation of rhodamine B (RhB) dye, phenol and bisphenol A (BPA) in aqueous solution, under visible light irradiation. An important improvement of the catalytic activity was observed using the nanocomposites as compared to ZTO solely. The photocatalytic enhancement can be attributed to a plasmonic effect at the interface between Ag@AgCl and ZTO. Thus, the good catalytic performance of the nanocomposites combined with their simple synthesis could provide a facile way to achieve highly efficient photocatalysts.
1. Introduction
In the last decade, a huge body of research has been devoted to the design and development of metal-semiconductor heterostructures due to their potential applications in optoelectronics, sensors, medical diagnostics, biosensing, solar energy conversion, and photocatalysis.1–9 In metal-semiconductor heterostructures, electronic mixing between the electronic states of the metal and semiconductor takes place.10 As a result, two types of electron transfer pathways are usually found for fused metal-semiconductor structures: (i) electrons can transfer from the metal to the semiconductor, (ii) photogenerated electrons in the semiconductor can be transferred to the metal.11,12 Either way, the electron transfer leads to increasing light absorption, promoting charge separation and transportation, prolonging their life-time, and enhancing redox reactions.2,13–17 The transferred electrons diffuse to the surface of the semiconductor materials and promote the formation of superoxide radical anions and hydroxyl radicals. Hybridization of metals and semiconductors enhances the physicochemical properties of semiconductors with wide band gap.9,18–20 To date, various metal-semiconductor hybrid materials have been prepared with the aim to improve their photocatalytic activity, because they can strongly absorb visible light through the localized surface plasmon resonance (LSPR). Ghosh et al.2 have demonstrated the good performance of Ag/WO3−x heterostructures for the photodegradation of methylene blue (MB) and RhB dyes under visible irradiation. Similarly, it has been shown that Ag decoration of oxide nanoparticles such as Ag–Bi2O3 nanospheres,21 Ag–SnO2,22 Ag–ZnO7,14–16,23,24 and Ag–TiO2 (ref. 9, 20, 25–27) induced a good improvement of their photocatalytic activity upon visible light irradiation.28 Furthermore, Ag/AgX (Cl, Br, I) was widely used to enhance semiconductor photocatalytic performance and represents a convenient way to hinder charge recombination.29–35 For example, it has been demonstrated that Ag/AgClQDs and Ag/AgBr sensitized Bi2WO6 exhibited enhanced photocatalytic degradation under visible light irradiation.34,35Recently, the photocatalytic properties of ZTO NPs for the degradation of organic pollutants have been investigated under UV illumination.36–39 Up to date, a limited progress has been made in the area of visible light-active ZTO; there is only one example on sun light driven photodegradation of Reactive Red 141.40 In a recent work, we have successfully applied ZTO NPs as an effective photocatalyst for the degradation of RhB under visible light irradiation and examined the influence of irradiation time on the photocatalytic process.41 Also, it has been demonstrated that ZTO-based heterostructures such as BiO/ZTO and g-C3N4/ZTO display an efficient charge transfer at the interface and enhanced the photo-induced charge separation under visible light irradiation.42,43
In continuation of our ongoing work on ZTO NPs photocatalysis41,44 with the aim to improve their performance, we report herein on the photocatalytic activity of Ag@AgCl/ZTO nanocomposites, with different Ag contents, for the photodegradation of RhB, phenol and bisphenol A under visible light irradiation. Accordingly, the parameters that influence the photocatalytic activity of Ag@AgCl/ZTO nanocomposite were investigated through analyzing its structural and optical properties. To the best of our knowledge, the visible light-driven photocatalytic activity of Ag@AgCl/ZTO nanocomposites for organic pollutants degradation has not been reported to date.
2. Experimental section
2.1. Reagents
All chemicals and reagents used for experiments and analyses were of analytical grade. Tin(IV) chloride pentahydrate (SnCl4·5H2O), zinc acetate dehydrate [Zn(CH3COO)2·2H2O], sodium hydroxide (NaOH), silver nitrate (AgNO3), ethylene glycol (EG), rhodamine B (RhB), ammonium oxalate (AO), isopropanol (IPA), benzoquinone (BQ), bisphenol A (BPA) and phenol were purchased from Sigma-Aldrich and used as received without further purification.2.2. Synthesis of Ag@AgCl/ZTO
In a typical process, 0.004 mol of Zn(CH3COO)2·2H2O and 0.002 mol of SnCl4·5H2O were dissolved separately in 8 cm3 of deionized water under continuous stirring at room temperature. The two solutions were mixed together, then NaOH aqueous solution (1 M) was added dropwise to the above mixture to form a white precipitate at pH = 8 and the solution was stirred for 30 min. The resulting solution was transferred into a Teflon-lined stainless steel autoclave (50 cm3 capacity) and kept at 200 °C for 16 h. Subsequently, the autoclave was cooled to ambient temperature. A 10 cm3 of ethylene glycol and 5 cm3 of 0.1 M silver nitrate aqueous solution were added into the suspension and the reaction was further kept at 200 °C for 8 h. A yellow precipitate was obtained, separated by centrifugation (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), washed repeatedly with deionized water (5 times) and finally dried at 60 °C for 24 h. We refer to the prepared Ag@AgCl/ZTO nanocomposites with 0 g L−1, 0.003 g L−1 and 0.1 g L−1 AgNO3 suspension in the starting solution as ZTO, Z-Ag1, and Z-Ag2, respectively.
000 rpm), washed repeatedly with deionized water (5 times) and finally dried at 60 °C for 24 h. We refer to the prepared Ag@AgCl/ZTO nanocomposites with 0 g L−1, 0.003 g L−1 and 0.1 g L−1 AgNO3 suspension in the starting solution as ZTO, Z-Ag1, and Z-Ag2, respectively.
Ag NPs/ethylene glycol (Ag/EG) were also synthesized using the same protocol. Briefly, a mixture of 20 cm3 of ethylene glycol and 10 cm3 of 0.1 M silver nitrate aqueous solution was transferred into a Teflon-lined stainless steel autoclave (50 cm3 capacity) and heated at 200 °C for 8 h. A yellow precipitate was obtained, separated by centrifugation (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm), washed repeatedly with deionized water (5 times) and finally dried at 60 °C for 24 h.
000 rpm), washed repeatedly with deionized water (5 times) and finally dried at 60 °C for 24 h.
2.3. Characterization
The crystal phase of obtained powders was analyzed by X-ray diffraction (XRD) patterns using an X'pert Pro X-ray diffractometer with Cu Kα1 radiation, λ = 0.15406 nm in the 2θ range of 5–70°, 0.033° as increment, integration time 50 s.Scanning electron microscopic images of the nanocomposites were obtained using an electron microscope ULTRA 55 (Zeiss) equipped with a thermal field emission emitter, three different detectors (EsB detector with filter grid, high efficiency In-lens SE detector, Everhart-Thornley Secondary Electron Detector) and an X-ray energy dispersive analysis device (EDX analysis) (Bruket AXS). An accelerating voltage between 5 kV (nitrogen detection) and 15 kV was used.
Transmission electron microscopy (TEM) imaging was performed with a Philips CM30 microscope operating at 300 kV. It was equipped with a Gatan SS CCD camera and a Digital Micrograph software for the acquisition of electron diffraction patterns, bright field and high-resolution imaging.
Absorption spectra were performed using a Perkin Elmer Lambda UV-vis 950 spectrophotometer in the 250–800 nm wavelength range. The reflectance spectra were recorded using the Angle Absolute Universal Reflectance Accessory (URA) purchased from Perkin Elmer.
X-ray photoelectron spectroscopy (XPS) measurements were performed with an ESCALAB 220 XL spectrometer from vacuum generators featuring a monochromatic Al Kα X-ray source (1486.6 eV) and a spherical energy analyzer operated in the CAE (constant analyzer energy) mode (CAE = 100 eV for survey spectra and CAE = 40 eV for high-resolution spectra), using the electromagnetic lens mode. The detection angle of the photoelectrons is 30°, as referenced to the sample surface. After subtraction of the Shirley-type background, the core-level spectra were decomposed into their components with mixed Gaussian–Lorentzian (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) shape lines using the Casa XPS software. Quantification calculations were performed using sensitivity factors supplied by PHI.
70) shape lines using the Casa XPS software. Quantification calculations were performed using sensitivity factors supplied by PHI.
The photoluminescence (PL) spectra were recorded at room temperature in the 350–900 nm spectral range using an argon laser with a wavelength of 333.6 nm as the excitation source.
2.4. Photocatalytic experiments
The photocatalytic activity of Ag@AgCl/ZTO nanocomposites for the degradation of RhB dye was evaluated under visible light irradiation. In a typical procedure, 1 mg of as-synthesized Ag@AgCl/ZTO catalyst was added into 2 cm3 aqueous solution of RhB (5 × 10−6 mol L−1) under constant stirring. Prior to visible light irradiation, a dark adsorption experiment was performed to achieve adsorption equilibrium between RhB dye and photocatalyst. Then, the above suspension was exposed to visible light irradiation at room temperature in air through with a cut off filter (λ = 420 nm, to suppress the light with wavelength shorter than 420 nm) using a visible fiber lamp (Spot Light Source 400–700 nm, L9566-03, Hamamatsu, Japan) under constant stirring. The intensity of the light was measured using a PM600TM Laser Fiber Power Meter (Coherent Inc., USA) and was determined as being 0.5 W cm−2. The concentration of RhB was monitored by UV-vis spectrophotometer at 554 nm.45,46Similarly, the photodegradation of phenol and bisphenol A (BPA) was also performed using Ag@AgCl/ZTO catalyst. For this study, 2 cm3 of 5 × 10−4 mol L−1 phenol aqueous (or BPA) solution and 0.5 g L−1 catalyst were mixed in a quartz cuvette. Then, the stable aqueous solution was irradiated with the same visible light lamp (λ > 420 nm, 0.5 W cm−2).
3. Results and discussion
3.1. Structure, morphology and chemical composition of Ag@AgCl/ZTO nanocomposites
The XRD patterns of pure Zn2SnO4 (ZTO) and Ag@AgCl/ZTO nanocomposites (Z-Ag1 and Z-Ag2) are depicted in Fig. 1. Pure ZTO shows diffraction peaks at 2θ values of 17.74°, 29.17°, 34.35° and 35.93° attributed to (111), (220), (311) and (222) planes of face centered cubic (JCPDS file no. 01-074-2184), respectively. All the as-prepared Z-Ag samples exhibit two set of diffraction peaks relative to ZTO and AgCl phases. The peaks at 2θ values of 27.83°, 32.24°, 46.23° and 57.48° correspond to (111), (200), (220) and (222) planes of face centered cubic silver chloride (JCPDS file no. 00-031-1238), respectively. It should be noted here that no characteristic peaks that belong to Ag were detected. The reason may be that the amount of Ag NPs produced on the surface of the ZTO NPs is too low to be detected.Fig. 2 illustrates a typical scanning electron microscopy (SEM) image of Z-Ag2 sample; one can easily observe the appearance of spherical nanoparticles of ZTO assembled with Ag@AgCl species. It can be seen that Z-Ag2 was constituted of irregular particles with the size of about 45 ± 2 nm. The big particles could be attributed to aggregated particles.
EDX analysis of Z-Ag2 shows the characteristics elements (Zn, Sn, O, Ag, Cl) of the nanocomposite material (Fig. 2). The Si in the EDX spectrum originates from crystalline silicon onto which the composite was deposited for SEM/EDX analysis.
The morphology of Ag@AgCl/ZTO nanohybrid structure was further characterized using transmission electron microscopy (TEM) imaging (Fig. 3). It is clearly seen that the Ag@AgCl species with ∼200 nm in size are distributed in the ZTO matrix. As depicted in the EDX mapping, one could clearly see the Zn, Sn, O and Ag elements distribution within the selected area. The EDX elemental mapping results also confirm the heterostructure of the selected sample. Several particles of Ag@AgCl are surrounded by the yellow circles.
X-ray photoelectron spectroscopy (XPS) analysis was performed to determine the surface chemical composition and state of Ag@AgCl/ZTO nanocomposites. Fig. 4 displays the XPS survey spectrum of Z-Ag2 sample. It comprises peaks due mainly to Zn, Sn, Cl, Ag, O and C. The carbon is most likely due to residual carbon from ethylene glycol or contamination during sample handling and storage.
 | ||
| Fig. 4 X-ray photoelectron spectroscopy (XPS) survey spectrum of Z-Ag2. The inset corresponds to a zoom in the 0–400 eV range. | ||
The core level XPS spectrum of Zn 2p depicts two peaks at 1020.0 and 1043.1 eV attributed to Zn 2p1/2 and Zn 2p3/2, respectively, in accordance with the Zn2+ oxidation state of Zn (Fig. 5A). Fig. 5B shows that the Sn 3d spectrum can be fitted by two strong energy bands centered at 484.7 and 493.1 eV, which are ascribed to the 3d5/2 and 3d3/2 spin orbits, respectively with a peak separation of 8.4 eV. Fig. 5C exhibits the core level spectrum of Ag 3d species in Ag@AgCl/ZTO nanocomposite, showing two individual peaks at 365.5 eV for Ag 3d5/2 and at 371.8 eV for Ag 3d3/2 with 6.3 eV splitting between the two peaks, suggesting the presence of ionic silver. These two peaks could be further deconvoluted into sets of peaks at 365.7, 366.68 eV, and 371.7, 372.7 eV, respectively. The standard binding energy of Ag 3d5/2 for metallic Ag is at 368.2 eV,47 which is different from our result; this shift may be explained by a strong interaction between Ag and AgCl. On the other hand, XPS analysis (Fig. 5A) shows an inequality in the surface atomic ratio of silver to chlorine.32 Indeed, the ratio of Ag/Cl (>1) is much higher than the stoichiometric ratio (1/1) in AgCl, which can be attributed to excessive Ag on the surface of Ag@AgCl/ZTO. The XPS core level spectra of Zn 2p (1020 and 1043.1 eV) and Sn 3d (484.7 and 493.1 eV) showed that their binding energies in Ag@AgCl/ZTO nanocomposite were slightly different, suggesting a strong interaction between the Ag@AgCl and ZTO NPs (Fig. 5A and B).
Once the Ag@AgCl nanospecies are deposited on the ZTO surface, electron transfer occurs from Ag@AgCl to the conduction band (CB) of ZTO uncovering positive ions at their place because the work function of Ag is different from that of ZTO semiconductor. Therefore, a new Fermi energy level in Ag@AgCl/ZTO is formed, indicating a strong interaction between silver and the ZTO NPs.9
3.2. Optical properties of Ag@AgCl/ZTO nanocomposites
UV-vis absorption spectra of Ag@AgCl/ZTO nanocomposites are displayed in Fig. 6. Ag@AgCl/ZTO nanocomposites exhibit two UV absorption bands at 362 and 440 nm. The band at 362 nm corresponds to the band gap energy of ZTO NPs, estimated to be 3.46 eV (ref. 41) (Fig. S1†), while the band at 440 nm is due to surface plasmon, which further confirms that silver had been deposited successfully on the surface of ZTO. The low absorption of Z-Ag1 suggests that the density of Ag@AgCl is lower in this sample as compared to Z-Ag2. The wavelength distribution of the absorbed light is an important property of photocatalysts.On the basis of the above analysis, a possible formation mechanism of the Ag@AgCl/ZTO was proposed. In a typical system, ethylene glycol was successively added to ZTO to form the host solution. Then, AgNO3 was injected into the above solution. The formation of Ag@AgCl can be explained as follow: on the one hand, when Ag+ cations are dispersed in an aqueous solution, part of them can be reduced to Ag0 in the presence of ethylene glycol.48 On the other hand, the Cl− anions from the host water phase (the dissolution of SnCl4·5H2O produces Cl− anions in the solution) can collide with the Ag+ cations and form AgCl nanostructures.
In detail, Ag@AgCl particles can transfer electrons to ZTO in Ag@AgCl/ZTO heterostructures after Fermi level alignment. This transfer will result in a deficit of electrons on the surface of Ag@AgCl nanostructures, leading to a red shift in the surface plasmon absorption which could contribute to the photocatalytic activity in the visible light range. Similar results have been reported elsewhere for anchoring Ag clusters and Ag@AgCl on semiconductor surfaces with high enhancement of the photocatalytic activity.23,31,33,49
Taken together, the localized surface plasmon observed in UV-vis and the XPS results could show some evidence for the coexistence of Ag0 and AgCl species in the sample.
Fig. 7 exhibits the room-temperature photoluminescence (PL) spectra of Ag@AgCl/ZTO heterostructures. All individual curves show similar visible emission centered at around 630 nm. The PL measurements were performed to determine the charge recombination and migration efficiency of the ZTO and Ag@AgCl/ZTO nanocomposites, because the photocatalytic activity is closely linked to the PL intensity and the recombination rate of photo-excited electrons and holes.
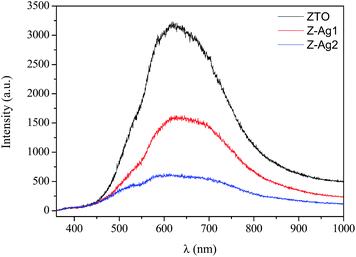 | ||
| Fig. 7 Room temperature photoluminescence spectra of ZTO NPs (black), Z-Ag1 (red) and Z-Ag2 (blue) nanocomposites using an excitation wavelength of 333.6 nm. | ||
The PL intensity of Ag@AgCl/ZTO nanohybrids was lower than that of pure ZTO NPs and it decreases with increasing the Ag content. This suggests that anchoring of Ag@AgCl species to the ZTO surface can result in quenching of the ZTO NPs PL as well as the enhanced pump absorption by the plasmon oscillation. The lowering of the PL intensity indicates an inhibition of electron–hole radiative recombination, which leads to a longer lifetime of the photogenerated carriers. Therefore, a possible energy transfer between different active centers, with increasing silver content, could be at the origin of the PL quenching. In general, the efficient charge separation and inhibited electron–hole recombination by Ag@AgCl nanostructures is a feasible way for enhancing the photocatalytic activity of metal oxide NPs.23,31,33,50
3.3. Photocatalytic activity of Ag@AgCl/ZTO nanocomposites
The fact that RhB is active to visible light, its photodegradation may be caused by a dye-sensitized path, which does not require the band gap excitation of a semiconductor. To rule out this possibility and further identify the visible light activity of Ag@AgCl/ZTO catalyst, we also tested the degradation of phenol and bisphenol A (BPA) that have no absorption in the visible light region. Fig. 8(A and B) and S4† show the UV-vis absorption spectra of RhB, phenol and bisphenol A (BPA) aqueous solution in the presence of Z-Ag2 catalyst. Under visible light irradiation and in absence of catalyst, the absorbance spectrum of RhB does not show a significant change, which confirms the stability of the dye under our experimental conditions (Fig. S2†). However, in the presence of the Z-Ag2 catalyst, a progressive decrease of the RhB absorption intensity (at 554 nm) with increasing irradiation time can be observed. There are no new peaks during the photocatalytic reaction process, indicative of the degradation of possible intermediate products. ZTO NPs demonstrated the low photocatalytic activity. In fact, after 120 min of irradiation, ZTO NPs degrade 85% of RhB (Fig. 8C, blue curve) and no appreciable degradation is seen for phenol (Fig. 8D, blue curve). In the presence of Ag/E gas catalyst (without ZTO), no degradation of phenol has been observed (Fig. 8D, red curve).Significantly higher percentages of RhB, phenol and bisphenol A (BPA) are degraded after loading Ag@AgCl species on ZTO NPs. Nearly, 100% of RhB is degraded in the presence of Z-Ag1 catalyst after 120 min irradiation. The best results were obtained for Z-Ag2 sample with an almost complete degradation of RhB and phenol (∼100%) after 25 min and 120 min of irradiation, respectively. Moreover, the photocatalytic performance of the Z-Ag2 catalysts distinctly increases with increasing the weight concentration. 1 g L−1 of Z-Ag2 nanocomposite shows the highest activity: RhB is degraded almost completely within 15 min (Fig. 8C and S3†). The photocatalytic activity of Ag@AgCl/ZTO (Z-Ag2) composite was further assessed for the degradation of bisphenol A (Fig. S4†). The concentration of BPA decreased by 38% after 5 h under visible light irradiation in the presence of Z-Ag2 catalyst.
It has been proven that the photocatalytic degradation of RhB dye follows pseudo-first order kinetics and the rate constant can be determined by the following relation:51
 | (1) |
The apparent rate constant k (in min−1) was calculated from the graph of 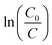 versus time interval, where C0 is the concentration of dye at equilibrium established under dark conditions and C is the concentration of nondegraded dye at different time intervals of irradiation. The Z-Ag2 nanocomposite demonstrated the much higher rate constant with a value of 0.13 min−1 much ahead of its counterpart ZTO NPs with a value of 0.01 min−1 (Fig. 9A).
versus time interval, where C0 is the concentration of dye at equilibrium established under dark conditions and C is the concentration of nondegraded dye at different time intervals of irradiation. The Z-Ag2 nanocomposite demonstrated the much higher rate constant with a value of 0.13 min−1 much ahead of its counterpart ZTO NPs with a value of 0.01 min−1 (Fig. 9A).
The low PL emission intensity of Z-Ag2 compared to that of ZTO NPs confirms the efficiency of charge separation at the interface of Ag@AgCl/ZTO nanocomposite, consistent with the photocatalytic results.
To investigate the stability of Z-Ag2, the recycled experiments for the degradation of RhB were performed in the presence of 0.5 g L−1 of catalyst under visible light irradiation (Fig. 9B). After each run, a fresh concentrated solution of RhB (4.8 μg per run) was added to obtain the initial concentration of RhB (5 × 10−6 mol L−1). The recycled usage did not affect the Z-Ag2 catalytic activity even after six runs, which exhibited good performance and stability during all the runs. These results suggest that Z-Ag2 catalyst was stable and had a high recyclability for RhB degradation.
In order to further confirm the photocatalytic mechanism of the charge transfer in Ag@AgCl/ZTO composites, we also used a series of trapping experiments to determine the main active species responsible for the degradation of RhB under visible light irradiation.35,52 Herein, ammonium oxalate (AO), isopropanol (IPA) and benzoquinone (BQ) were used as hole (h+), hydroxyl radical (OH˙) and superoxide radical (O2˙−) scavengers, respectively. As shown in Fig. S5,† after addition of BQ, the photocatalytic performance was slightly decreased. However, in the presence of isopropanol (IPA) and ammonium oxalate (AO) the photocatalytic degradation of RhB with the Z-Ag2 sample was not affected. Therefore, we can postulate that electrons are the reactive species.
Based on the PL measurement and previous reports on Ag@AgCl anchored on the surface of semiconductor catalysts, a possible photocatalytic mechanism for Ag@AgCl/ZTO nanocomposites is proposed in Scheme 1. Ag nanospecies can absorb the visible light and convert of incidents photons into LSPR oscillations, which facilitate the separation of photo-generated electrons at the surface of Ag nanospecies. On the other hand, AgCl and ZTO cannot be excited under visible light irradiation owing to their large band gaps,30,50 thus the energy position of ZTO is favorable to receive electrons from the Ag nanospecies.
 | ||
| Scheme 1 Schematic diagram illustrating the proposed charge transfer in Ag@AgCl/ZTO system under visible light illumination. | ||
Then, the excited electrons have enough energy and can transfer to the CB of AgCl and ZTO. So, the injected electrons react with O2 molecules adsorbed on AgCl and ZTO surface to yield O2−˙ radical anion; interaction with H2O or OH− produces ˙OH radical species. The superoxide radical and OH˙ radicals are very efficient for the degradation of organic pollutants.29–33,42 Indeed, anchoring Ag@AgCl nanospecies on the surface of ZTO NPs could increase the availability of active sites and provides a large number of interfaces for the transfer of photoinduced charge carriers, which could form a hierarchical nanostructure for the degradation of organic pollutants.
4. Conclusion
In summary, Ag@AgCl/ZTO nanocomposites were synthesized by a facile hydrothermal method. Compared to ZTO NPs, the Ag@AgCl/ZTO nanocomposites showed a significant improvement of visible-light photocatalytic activity for the degradation of RhB and phenol. The Ag@AgCl anchored on the surface of the ZTO shows strong plasmonic effect that could effectively hinder the recombination of charge carriers at the interface. The PL and UV-vis measurements illustrated that the synergistic effect between Ag@AgCl and ZTO was very important for the enhancement of the photoactivity of Ag@AgCl/ZTO. This study provides a facile way to achieve highly efficient photocatalysts, which could be used to develop plasmon-based substrates for improving photocatalytic performance in water.Acknowledgements
The authors gratefully acknowledge financial support from the Centre National de Recherche Scientifique (CNRS), the Université Lille 1, the Nord Pas de Calais region, and the CMCU through the PHC-Utique N°13G 1302 program. The TEM facility in Lille (France) is supported by the Conseil Régional du Nord-Pas-de Calais, and the European Regional Development Fund (ERDF).References
- U. Banin, Y. Ben-Shahar and K. Vinokurov, Hybrid Semiconductor–Metal Nanoparticles: From Architecture to Function, Chem. Mater., 2013, 26, 97–110 CrossRef.
- S. Ghosh, M. Saha, S. Paul and S. K. De, Maximizing the Photo Catalytic and Photo Response Properties of Multimodal Plasmonic Ag/WO3−X Heterostructure Nanorods by Variation of the Ag Size, Nanoscale, 2015, 7, 18284–18298 RSC.
- Y. Nishijima, K. Ueno, Y. Yokota, K. Murakoshi and H. Misawa, Plasmon-Assisted Photocurrent Generation from Visible to near-Infrared Wavelength Using a Au-Nanorods/TiO2 Electrode, J. Phys. Chem. Lett., 2010, 1, 2031–2036 CrossRef CAS.
- K. P. Acharya, R. S. Khnayzer, T. O'Connor, G. Diederich, M. Kirsanova, A. Klinkova, D. Roth, E. Kinder, M. Imboden and M. Zamkov, The Role of Hole Localization in Sacrificial Hydrogen Production by Semiconductor–Metal Heterostructured Nanocrystals, Nano Lett., 2011, 11, 2919–2926 CrossRef CAS PubMed.
- M. Berr, A. Vaneski, A. S. Susha, J. Rodríguez-Fernández, M. Dubliner, F. Jäckel, A. L. Rogach and J. Feldmann, Colloidal Cds Nanorods Decorated with Subnanometer Sized Pt Clusters for Photocatalytic Hydrogen Generation, Appl. Phys. Lett., 2010, 97, 093108 CrossRef.
- J. Li and N. Wu, Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review, Catal. Sci. Technol., 2015, 5, 1360–1384 CAS.
- X. Liu, W. Li, N. Chen, X. Xing, C. Dong and Y. Wang, Ag–ZnO Heterostructure Nanoparticles with Plasmon-Enhanced Catalytic Degradation for Congo Red under Visible Light, RSC Adv., 2015, 5, 34456–34465 RSC.
- Y. Shemesh, J. E. Macdonald, G. Menagen and U. Banin, Synthesis and Photocatalytic Properties of a Family of CdS–Pdx Hybrid Nanoparticles, Angew. Chem., 2011, 123, 1217–1221 CrossRef.
- V. Subramanian, E. E. Wolf and P. V. Kamat, Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration, J. Am. Chem. Soc., 2004, 126, 4943–4950 CrossRef CAS PubMed.
- D. Steiner, T. Mokari, U. Banin and O. Millo, Electronic Structure of Metal-Semiconductor Nanojunctions in Gold CdSe Nanodumbbells, Phys. Rev. Lett., 2005, 95, 056805 CrossRef CAS PubMed.
- S. K. Dutta, S. K. Mehetor and N. Pradhan, Metal Semiconductor Heterostructures for Photocatalytic Conversion of Light Energy, J. Phys. Chem. Lett., 2015, 6, 936–944 CrossRef CAS PubMed.
- J. Dana, T. Debnath, P. Maity and H. N. Ghosh, Enhanced Charge Separation in an Epitaxial Metal-Semiconductor Nanohybrid Material Anchored with an Organic Molecule, J. Phys. Chem. C, 2015, 119, 22181–22189 CAS.
- L. Carbone, A. Jakab, Y. Khalavka and C. Sönnichsen, Light-Controlled One-Sided Growth of Large Plasmonic Gold Domains on Quantum Rods Observed on the Single Particle Level, Nano Lett., 2009, 9, 3710–3714 CrossRef CAS PubMed.
- S. Kandula and P. Jeevanandam, Sun-Light-Driven Photocatalytic Activity by ZnO/Ag Heteronanostructures Synthesized Via a Facile Thermal Decomposition Approach, RSC Adv., 2015, 5, 76150–76159 RSC.
- R. Georgekutty, M. K. Seery and S. C. Pillai, A Highly Efficient Ag-ZnO Photocatalyst: Synthesis, Properties, and Mechanism, J. Phys. Chem. C, 2008, 112, 13563–13570 CAS.
- S. Kuriakose, V. Choudhary, B. Satpati and S. Mohapatra, Facile Synthesis of Ag–ZnO Hybrid Nanospindles for Highly Efficient Photocatalytic Degradation of Methyl Orange, Phys. Chem. Chem. Phys., 2014, 16, 17560–17568 RSC.
- L. Wu, F. Li, Y. Xu, J. W. Zhang, D. Zhang, G. Li and H. Li, Plasmon-Induced Photoelectrocatalytic Activity of Au Nanoparticles Enhanced TiO2 Nanotube Arrays Electrodes for Environmental Remediation, Appl. Catal., B, 2015, 164, 217–224 CrossRef CAS.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Visible Light Driven Type II Heterostructures and Their Enhanced Photocatalysis Properties: A Review, Nanoscale, 2013, 5, 8326–8339 RSC.
- X. Yu, J. Liu, A. Genç, M. Ibáñez, Z. Luo, A. Shavel, J. Arbiol, G. Zhang, Y. Zhang and A. Cabot, Cu2ZnSnS4–Ag2S Nanoscale P–N Heterostructures as Sensitizers for Photoelectrochemical Water Splitting, Langmuir, 2015, 31, 10555–10561 CrossRef CAS PubMed.
- S.-W. Lee, S. Obregón and V. Rodríguez-González, The Role of Silver Nanoparticles Functionalized on TiO2 for Photocatalytic Disinfection of Harmful Algae, RSC Adv., 2015, 5, 44470–44475 RSC.
- X. Zhong, Z. Dai, F. Qin, J. Li, H. Yang, Z. Lu, Y. Liang and R. Chen, Ag-Decorated Bi2O3 nanospheres with Enhanced Visible-Light-Driven Photocatalytic Activities for Water Treatment, RSC Adv., 2015, 5, 69312–69318 RSC.
- S. A. Ansari, M. M. Khan, M. O. Ansari, J. Lee and M. H. Cho, Visible Light-Driven Photocatalytic and Photoelectrochemical Studies of Ag–SnO2 Nanocomposites Synthesized Using an Electrochemically Active Biofilm, RSC Adv., 2014, 4, 26013–26021 RSC.
- S. G. Kumar and K. S. R. K. Rao, Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications, RSC Adv., 2015, 5, 3306–3351 RSC.
- S. S. Patil, M. G. Mali, M. S. Tamboli, D. R. Patil, M. V. Kulkarni, H. Yoon, H. Kim, S. S. Al-Deyab, S. S. Yoon, S. S. Kolekar and B. B. Kale, Green Approach for Hierarchical Nanostructured Ag-ZnO and Their Photocatalytic Performance Under Sunlight, Catal. Today, 2016, 260, 126–134 CrossRef CAS.
- C. Jia, H.-S. Chen and P. Yang, Selective Growth of TiO2 Beads on Ag Nanowires and Their Photocatalytic Performance, CrystEngComm, 2015, 17, 4895–4902 RSC.
- L. Yu, X. Yang, Y. Ye, X. Peng and D. Wang, Silver Nanoparticles Decorated Anatase TiO2 Nanotubes for Removal of Pentachlorophenol from Water, J. Colloid Interface Sci., 2015, 453, 100–106 CrossRef CAS PubMed.
- Z. Zhao, Y. Wang, J. Xu and Y. Wang, Mesoporous Ag/TiO2 Nanocomposites with Greatly Enhanced Photocatalytic Performance Towards Degradation of Methyl Orange under Visible Light, RSC Adv., 2015, 5, 59297–59305 RSC.
- L. Zhu, C. He, Y. Huang, Z. Chen, D. Xia, M. Su, Y. Xiong, S. Li and D. Shu, Enhanced Photocatalytic Disinfection of E. Coli 8099 Using Ag/BiOI Composite Under Visible Light Irradiation, Sep. Purif. Technol., 2012, 91, 59–66 CrossRef CAS.
- X. Yao, X. Liu and X. Hu, Synthesis of the Ag/AgCl/g-C3N4 Composite With High Photocatalytic Activity Under Visible Light Irradiation, ChemCatChem, 2014, 6, 3409–3418 CrossRef CAS.
- X. Li, S. Fang, L. Ge, C. Han, P. Qiu and W. Liu, Synthesis of Flower-Like Ag/AgCl-Bi2MoO6 Plasmonic Photocatalysts With Enhanced Visible-Light Photocatalytic Performance, Appl. Catal., B, 2015, 176–177, 62–69 CrossRef CAS.
- A. Meng, J. Xing, Z. Li, Q. Wei and Q. Li, Ag/AgCl/ZnO Nano-Networks: Preparation, Characterization, Mechanism and Photocatalytic Activity, J. Mol. Catal. A: Chem., 2016, 411, 290–298 CrossRef CAS.
- H. Yin, X. Wang, L. Wang, Q. Nie, Y. Zhang, Q. Yuan and W. Wu, Ag/AgCl Modified Self-Doped TiO2 Hollow Sphere With Enhanced Visible Light Photocatalytic Activity, J. Alloys Compd., 2016, 657, 44–52 CrossRef CAS.
- J. Zhu, S. Liu, Q. Yang, P. Xu, J. Ge and X. Guo, Fabrication of Flower-Like Ag@AgCl/Bi2WO6 Photocatalyst and Its Mechanism of Photocatalytic Degradation, Colloids Surf., A, 2016, 489, 275–281 CrossRef CAS.
- Y. Liang, S. Lin, L. Liu, J. Hu and W. Cui, Oil-in-Water Self-Assembled Ag@AgCl QDs Sensitized Bi2WO6: Enhanced Photocatalytic Degradation under Visible Light Irradiation, Appl. Catal., B, 2015, 164, 192–203 CrossRef CAS.
- S. Lin, L. Liu, J. Hu, Y. Liang and W. Cui, Nano Ag@AgBr Surface-Sensitized Bi2 WO6 Photocatalyst: Oil-in-Water Synthesis and Enhanced Photocatalytic Degradation, Appl. Surf. Sci., 2015, 324, 20–29 CrossRef CAS.
- X. Fu, X. Wang, J. Long, Z. Ding, T. Yan, G. Zhang, Z. Zhang, H. Lin and X. Fu, Hydrothermal Synthesis, Characterization, and Photocatalytic Properties of Zn2SnO4, J. Solid State Chem., 2009, 182, 517–524 CrossRef CAS.
- W. Cun, W. Xinming, Z. Jincai, M. Bixian, S. Guoying, P. Ping'An and F. Jiamo, Synthesis, Characterization and Photocatalytic Property of Nano-Sized Zn2SnO4, J. Mater. Sci., 2002, 37, 2989–2996 CrossRef.
- E. L. Foletto, S. L. Jahn and R. de Fatima Peralta Muniz Moreira, Hydrothermal Preparation of Zn2SnO4 Nanocrystals and Photocatalytic Degradation of a Leather Dye, J. Appl. Electrochem., 2010, 40, 59–63 CrossRef CAS.
- X. Lou, X. Jia, J. Xu, S. Liu and Q. Gao, Hydrothermal Synthesis, Characterization and Photocatalytic Properties of Zn2SnO4 Nanocrystal, Mater. Sci. Eng., A, 2006, 432, 221–225 CrossRef.
- E. L. Foletto, J. M. Simoes, M. A. Mazutti, S. L. Jahn, E. I. Muller, L. S. Fagundes Pereira and E. M. de Moraes Flores, Application of Zn2SnO4 Photocatalyst Prepared by Microwave-Assisted Hydrothermal Route in the Degradation of Organic Pollutant Under Sunlight, Ceram. Int., 2013, 39, 4569–4574 CrossRef CAS.
- M. BenAli, F. Barka-Bouaifel, H. Elhouichet, B. Sieber, A. Addad, L. Boussekey, M. Férid and R. Boukherroub, Hydrothermal Synthesis, Phase Structure, Optical and Photocatalytic Properties of Zn2SnO4 Nanoparticles, J. Colloid Interface Sci., 2015, 457, 360–369 CrossRef CAS PubMed.
- T. Yan, H. Liu, M. Sun, X. Wang, M. Li, Q. Yan, W. Xu and B. Du, Efficient Photocatalytic Degradation of Bisphenol A and Dye Pollutants over BiOI/Zn2SnO4 Heterojunction Photocatalyst, RSC Adv., 2015, 5, 10688–10696 RSC.
- L. Zhang, X. Wang, Q. Nong, H. Lin, B. Teng, Y. Zhang, L. Zhao, T. Wu and Y. He, Enhanced Visible-Light Photoactivity of g-C3N4 via Zn2SnO4 Modification, Appl. Surf. Sci., 2015, 329, 143–149 CrossRef CAS.
- M. Ben Ali, H. H. Yolcu, H. Elhouichet, B. Sieber, A. Addad, L. Boussekey, M. Moreau, M. Férid, S. Szunerits and R. Boukherroub, Hydrothermal Synthesis of ZTO/Graphene Nanocomposite With Excellent Photocatalytic Activity Under Visible Light Irradiation, J. Colloid Interface Sci., 2016, 473, 66–74 CrossRef CAS PubMed.
- A. Barras, S. Cordier and R. Boukherroub, Fast Photocatalytic Degradation of Rhodamine B Over [Mo6Br8(N3)6]2− Cluster Units Under Sun Light Irradiation, Appl. Catal., B, 2012, 123–124, 1–8 CrossRef CAS.
- A. Barras, M. R. Das, R. R. Devarapalli, M. V. Shelke, S. Cordier and R. Boukherroub, One-Pot Synthesis of Gold Nanoparticle/Molybdenum Cluster/Graphene Oxide Composite and Its Photocatalytic Activity, Appl. Catal., B, 2013, 130–131, 270–276 CrossRef CAS.
- J. Thiel, L. Pakstis, S. Buzby, M. Raffi, C. Ni, D. J. Pochan and S. I. Shah, Antibacterial Properties of Silver-Doped Titania, Small, 2007, 3, 799–803 CrossRef CAS PubMed.
- C. Tian, W. Li, K. Pan, Q. Zhang, G. Tian, W. Zhou and H. Fu, One Pot Synthesis of Ag Nanoparticle Modified ZnO Microspheres in Ethylene Glycol Medium and Their Enhanced Photocatalytic Performance, J. Solid State Chem., 2010, 183, 2720–2725 CrossRef CAS.
- W. Zhou, T. Li, J. Wang, Y. Qu, K. Pan, Y. Xie, G. Tian, L. Wang, Z. Ren and B. Jiang, Composites of Small Ag Clusters Confined in the Channels of Well-Ordered Mesoporous Anatase TiO2 and Their Excellent Solar-Light-Driven Photocatalytic Performance, Nano Res., 2014, 7, 731–742 CrossRef CAS.
- B. Ma, J. Guo, W.-L. Dai and K. Fan, Ag-AgCl/WO3 Hollow Sphere with Flower-Like Structure and Superior Visible Photocatalytic Activity, Appl. Catal., B, 2012, 123–124, 193–199 CrossRef CAS.
- X. H. Wang, J. G. Li, H. Kamiyama, Y. Moriyoshi and T. Ishigaki, Wavelength-Sensitive Photocatalytic Degradation of Methyl Orange in Aqueous Suspension Over Iron(III)-doped TiO2 Nanopowders Under UV and Visible Light Irradiation, J. Phys. Chem. B, 2006, 110, 6804–6809 CrossRef CAS PubMed.
- Y. Tian, B. Chang, J. Lu, J. Fu, F. Xi and X. Dong, Hydrothermal Synthesis of Graphitic Carbon Nitride–Bi2WO6 Heterojunctions with Enhanced Visible Light Photocatalytic Activities, ACS Appl. Mater. Interfaces, 2013, 5, 7079–7085 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14813a |
| This journal is © The Royal Society of Chemistry 2016 |







