Molybdenum disulfide nanosheet embedded three-dimensional vertically aligned carbon nanotube arrays for extremely-excellent cycling stability lithium-ion anodes†
Haining Fana,
Xiaohua Chen*a,
Qunli Tanga,
Shanliang Chena,
Binbin Fana,
Aiping Hua,
Shiying Zhangb and
Yanghua Lib
aCollege of Materials Science and Engineering, Hunan University, Hunan Province Key Laboratory for Spray Deposition Technology and Application, Changsha 410082, China. E-mail: xiaohuachen@hnu.edu.cn; Fax: +86 73188823554; Tel: +86 73188821610
bHunan Province Key Laboratory of Applied Environmental Photocatalysis, Changsha University, Changsha 410022, China
First published on 16th August 2016
Abstract
Molybdenum disulfide (MoS2) nanosheets embedded in three-dimensional (3D) vertically aligned carbon nanotube arrays (VACNTs) have been fabricated via a simple nebulization-assisted hydrothermal method. The MoS2/VACNTs possess a highly ordered and uniformly oriented 3D structure with MoS2 nanosheets adhering strictly to the surface of VACNTs. When evaluated as lithium-ion anode materials, so-obtained MoS2/VACNTs composites containing 52 wt% MoS2 exhibit superb electrochemical performances, including high capacity (1078 mA h g−1 at 100 mA g−1 after 1st cycle), good rate capability (789 mA h g−1 at 2000 mA g−1 after 20 cycles), and extremely-excellent cycling stability, for the MoS2/VACNTs electrode can still deliver a discharge capacity of 512 mA h g−1 after 1000 cycles at 5000 mA g−1, compared with pristine MoS2 (negligible discharge capacity at the 70th cycle). Such high electrical properties can mainly be attributed to the unique well-directed pore-morphology which provides low-resistant shortest diffusion pathways upon the high-conductive VACNTs to accelerate ion/electron movement. Moreover, the elastic spare-space inside/outside VACNTs as a buffer factor effectively restrains large volumetric change from MoS2 during the charge/discharge process. It can be determined that such a structure is attractive to achieve extremely-excellent cycling stability lithium-ion anodes.
1 Introduction
Batteries, the major power sources for various portable electronic devices and hybrid electric vehicles, have already become the benchmark for the development level in advanced energy storage technology.1 Regardless of other alternatives, lithium-ion batteries (LIBs) are considered as the most promising renewable energy source due to their high energy density, long service life and unblemished safety record.2,3 Nevertheless, it must be pointed out that LIBs existing today have still not fulfilled their ultimate potential in either power densities or cycling perdurability. In these two aspects, the sorts and structures of electrode materials play a strategic role.4 Thereby, for the sake of meeting higher requirements of applications with ever-increasing energy demands, it is deeply critical to develop high-performance electrode materials to achieve next-generation strong-powering and long-lasting rechargeable LIBs.Over the past several decades, transition-mental sulfides (MSs) have been extensively studied in different areas, such as dry lubricants,5 solar cells,6,7 catalysis,8,9 super-capacitors,10 and field-effect transistors,11 etc. While used for LIBs anodes, MSs usually show higher theoretical lithium storage capacity than traditional commercial graphite anodes (372 mA h g−1).12,13 Furthermore, it is worth noting that active sites producing by MSs electrode would be more than those from transition-metal oxides during lithiation/delithiation process.12,14,15 As one of 2D layer-structured MSs, molybdenum disulfide (MoS2) prevailing in energy conversion and storage exhibits high theoretical capacity of 670 mA h g−1, excellent rate capability and low cost, for typically layered morphology enlarging its specific surface area and the weak van der Waals connecting between its layers facilitate Li+ migration during charging/discharging process.15–17 Furthermore, MoS2 itself contains a large amount and concentration of metallic 1T phases and short-range defects which can be electrochemically intercalated ions such as Li+, Na+ and K+ with extraordinary efficiency to further expand its practical capacity.18,19 Unfortunately, in the practice operation of lithium intercalation, it is typically difficult to achieve rapid transference for electrons between two adjoining MoS2 nanosheets, which could be ascribed to extremely limited conductivity in so-called c-direction and its intrinsic tendency to reaggregate or restack, thus leading to significant capacity loss.15,20 As well as, the MoS2 anodes experience a lower first-cycle coulombic efficiency and a shorter life-time induced by large volumetric change in the c-direction during Li+ insertion/extraction process.5,21 Worse still, huge volume change is likely to bring MoS2 pulverization problem which could accelerate capacity attenuation as well.
To overcome these problems, recent significant investigation drives hot-concern on the MoS2/C based nanocomposites. These composites mainly include few-layer MoS2 anchored on carbon nanosheet,15 MoS2 coated 3D graphene networks,12,22,23 and MoS2 grown on the surface of carbon nanotubes (CNTs) nanocomposites,24–26 etc. Hereinto, CNTs have been expected most profitable for electrode materials with excellent performance due to their original morphology: helically arranged graphitic layers, one-dimensional (1D) structured central canals and entanglement formation. These structure characteristics entail CNTs incorporated in composites serving as superb conductors with mechanical and chemical stability, high accessible surface area and excellent electrical conductivity.27,28 For instance, Shyamal K. Das fabricated carbon–CNT–MoS2 composites by one-step hydrothermal method and these composites retain a discharge capacity of 522 mA h g−1 with 98% columbic efficiency after 50 cycles at 100 mA g−1.29 Moreover, Junsong Chen reported MoS2 Nanosheets on CNTs backbone by glucose-assisted hydrothermal method and these MoS2/CNTs exhibit capacity up to 957 mA h g−1 after 1st cycle at 100 mA g−1, while after 60 cycles, a reversible capacity as high as 698 mA h g−1 can still be retained.24 However, these 1D CNTs randomly entangled provide only curved channels for ion migrating, which extend ion mobile distance and hinder electrolyte fast permeating. Therefore, it is extraordinarily significant to develop vertically aligned CNTs which possess one-direction oriented pore structures. These vertical pore structures could not only permit the electrolyte to efficiently permeate active materials but also facilitate Li+/electron transport by decreasing the diffusion distance. Meanwhile, the elastic spare-space inside/outside nanotubes can alleviate internal stress induced by the volume expansion/contraction of these electrochemically active phases.30
In this paper, we fabricate nitrogen doped VACNTs with high conductivity through chemical vapor deposition (CVD) technology using xylene as carbon source, cyclohexylamine as nitrogen source and ferrocene as iron catalyst. And then MoS2 nanosheets could be introduced into VACNTs forest through a simple hydrothermal method named nebulization-assisted infiltration using ammonium molybdate ((NH4)6Mo7O24·4H2O) and thiourea (CH4N2S) as sources, showed in Scheme 1. This process consists of two steps: one is using spray to produce small droplets which could be infiltrated into VACNTs due to gravity; the other is pyrolyzing above sources under 750 °C to synthesis MoS2: the (NH4)6Mo7O24 is decomposed to ultrathin MoOx, CH4N2S is pyrolyzed to H2S, and then, ultrathin MoOx nanosheets react with H2S to form few-layer MoS2 nanosheets.15 Moreover, we design three groups by spraying for 20, 30, and 40 minutes, in which MoS2 contents are 37.15%, 52%, and 59.9%, marked MoS2/VACNTs-1, MoS2/VACNTs-2, and MoS2/VACNTs-3, respectively. Plus, we synthesize pristine MoS2 as control group by hydrothermal method. Most importantly, their advanced electrical performance of LIBs effectively outperforms all the existing MoS2-based materials.
 | ||
| Scheme 1 Schematic illustration of the fabrication process for high-quality 3D structure-based MoS2/VACNTs. | ||
2 Materials and experimental methods
2.1. Preparation of MoS2 embedded into 3D vertically aligned CNTs
3D vertically aligned CNTs are synthesized via CVD method. Firstly, ferrocene (96.75 mM) was dissolved in mixed solution of xylene and cyclohexylamine (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 in the volumetric ratio) in a round bottom flask, while atomized using a KQ-300DE ultrasonic nebulizer (Kunshan Ultrasonic Instrument Co., Ltd., China) in an ultrasonic bath for 15 minutes. Secondly, completely-dissolved solution was atomized by the ultrasonic nebulizer and passed through a quartz tube (45 mm in internal diameter, 1000 mm in length), in which quartz slice (30 mm in length, 30 mm in width, 3 mm in height) was placed directly above thermocouple as the substrate to grow samples at 900 °C provided by an open vacuum/atmosphere tube furnace (SK-G05123K Tianjin Zhonghuan Experiment Electric Stove Co. Ltd., China) with argon flowing (750 sccm) as carrier gas for 60 minutes. When reaction completed, ultrasonic nebulizer could be stopped and argon flow regulated down to 150 sccm. Lastly, the above-synthesized sample would be permeated in nitric acid (70%, 30 mL) for 9 h at 120 °C to introduce oxygen active functional groups –COO– and –OH which can be used for the absorption of ionogenic groups,23 followed by deionized water washing until pH became neutral, and then was dried in vacuum at 80 °C overnight.31
35 in the volumetric ratio) in a round bottom flask, while atomized using a KQ-300DE ultrasonic nebulizer (Kunshan Ultrasonic Instrument Co., Ltd., China) in an ultrasonic bath for 15 minutes. Secondly, completely-dissolved solution was atomized by the ultrasonic nebulizer and passed through a quartz tube (45 mm in internal diameter, 1000 mm in length), in which quartz slice (30 mm in length, 30 mm in width, 3 mm in height) was placed directly above thermocouple as the substrate to grow samples at 900 °C provided by an open vacuum/atmosphere tube furnace (SK-G05123K Tianjin Zhonghuan Experiment Electric Stove Co. Ltd., China) with argon flowing (750 sccm) as carrier gas for 60 minutes. When reaction completed, ultrasonic nebulizer could be stopped and argon flow regulated down to 150 sccm. Lastly, the above-synthesized sample would be permeated in nitric acid (70%, 30 mL) for 9 h at 120 °C to introduce oxygen active functional groups –COO– and –OH which can be used for the absorption of ionogenic groups,23 followed by deionized water washing until pH became neutral, and then was dried in vacuum at 80 °C overnight.31
The synthesis scheme for MoS2 nanosheets embedded in the 3D VACNTs is as follows: 0.02 mmol mL−1 (NH4)6Mo7O24·4H2O and 0.25 mmol mL−1 CH4N2S were added to 40 mL deionized water in a round bottom flask followed by thoroughly mixed stirring. And then this dispersed even solution was displayed on the ultrasonic nebulizer which generated a mist of droplets infiltrating into above-obtained 3D structure VACNTs at room temperature with an argon carrying gas at 700 sccm. After nebulization, the resulting samples were heated 2 h at 750 °C under 5% H2/95% Ar at 150 sccm, converted to MoS2, followed by distilled water washing several times, finally drought at 60 °C under vacuum for 12 h. The detailed reaction equations (eqn (1)–(3)) are described as follows:15
| 3(NH4)6Mo7O24·4H2O → (14x − 24)NH3 + (21 − 7x)N2 + 21MoOx + (84 − 21x)H2O | (1) |
| CH4N2S + 2H2O → 2NH3 + H2S + CO2 | (2) |
| 4MoOx + (x + 6)H2S → 4MoS2 + (x − 2)SO3 + (x + 6)H2O | (3) |
Pristine MoS2 were synthesized through usually hydrothermal process: 0.35 g (NH4)6Mo7O24·4H2O and 0.35 g CH4N2S were dissolved in 40 mL distilled water and put in the autoclave at 180 °C by 12 h. After reaction, on cooling to room temperature, the sample was put in 50 °C ovens after filtering.
2.2. Materials characterizations
The crystal structures of all the samples were identified using X-ray diffraction (XRD) conducted on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation and the data collected from 10° to 80°. The morphologies were characterized by field-emission scanning electron microscope (SEM) at 5 kV on an S-4800 microscope equipment (Hitachi, Japan) and transmission electron microscope (TEM) images were obtained with a JEM-2100F TEM (JEOL, Japan) operating at 200 kV. Thermogravimetric (TG) analysis was carried out on a TG/DTA7300 thermal analyzer with a heating rate of 5 °C min−1 in flowing atmosphere from room temperature to 800 °C to calculate MoS2 content in each experiment.2.3. Electrochemical measurements
The electrochemical properties of the samples were measured using CR2025-type coin cells assembled in a high-purity-Ar filled glove box with lithium foils as the counter electrodes. Celgard 2300 microporous polypropylene membrane and 1 M LiPF6 in ethylene carbonate/dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) were used as the separator and electrolyte, respectively. The working electrode was prepared by mixing active material, polyvinylidene fluoride and carbon black with a weight ratio of 70
1 in volume) were used as the separator and electrolyte, respectively. The working electrode was prepared by mixing active material, polyvinylidene fluoride and carbon black with a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in N-methylpyrrolidone (NMP) with constant stirring for 6 h to form homogeneous slurry. The well mixed slurry was then spread on Cu foil and dried at 80 °C in a vacuum oven for 12 h. The charge–discharge tests were performed between 0.01 V and 3 V on a Land CT2001A batterytest system (Wuhan, China). Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation (CHI660E) in the frequency range from 100 kHz to 0.01 Hz with amplitude of 5.0 mV. Cyclic voltammetry (CV) profiles were carried out on CHI660E at a scanning rate of 0.1 mV s−1 between 0 V and 3.0 V (vs. Li/Li+).
10 in N-methylpyrrolidone (NMP) with constant stirring for 6 h to form homogeneous slurry. The well mixed slurry was then spread on Cu foil and dried at 80 °C in a vacuum oven for 12 h. The charge–discharge tests were performed between 0.01 V and 3 V on a Land CT2001A batterytest system (Wuhan, China). Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation (CHI660E) in the frequency range from 100 kHz to 0.01 Hz with amplitude of 5.0 mV. Cyclic voltammetry (CV) profiles were carried out on CHI660E at a scanning rate of 0.1 mV s−1 between 0 V and 3.0 V (vs. Li/Li+).
3 Results and discussion
3.1. Morphology and structure characterization
Fig. 1 shows the XRD patterns of VACNTs, pristine MoS2 and MoS2/VACNTs composites. The peaks at 2θ = 13.9°, 33.5°, 37.1° and 59.3° can be indexed to (002), (100), (103) and (110) planes of MoS2, and all the diffraction peaks agree well with characteristic peaks of MoS2 in previous valuable literature.29 It is worth noting that the strong peak at 26° could be ascribed to (002) reflection of the VACNTs.31 The XRD results confirm the presence of MoS2 embedded in VACNTs and exhibit high purity of MoS2 in these composites compared to the pristine one because of no significant differences between them in the XRD patterns besides (002) reflection. In addition, there are different diffraction intensities of MoS2 characteristic peaks, meaning various levels of content in different experimental groups, which could correspond with TG analysis undersides.The SEM images of 3D VACNTs, pristine MoS2 and MoS2/VACNTs composites are shown in Fig. 2. Fig. 2a and b present the morphological characterization of 3D-matrix VACNTs forest. This forest structure is consisted of one-direction oriented carbon nanotubes with approximate 80 nm in diameters. As showed in Fig. 2c–e, the MoS2 nanosheets are adhered to the surface of the VACNTs strictly and homogeneously owing to the interaction between oxygen containing functional groups and MoS2 nanosheets,31 and the content of MoS2 increases as the nebulization time increases. Particularly, these samples still keep vertical and well dispersed compared with VACNTs, which proves this hydrothermal method with the help of nebulization-assisted infiltration is feasible. Fig. 2f indicate that the pristine MoS2 synthesized by hydrothermal method shows a flower-like shape and these flowers are stacked of MoS2 nanosheets. From TEM we can further ascertain the whole MoS2 nanosheets stack and wrap on the surface of VACNTs and so-formed layers of active materials has a uniform distribution as shown in Fig. 3a and b (Fig. S1†). The C, Mo and S element mappings (Fig. 3c–e) of the as-formed 3D MoS2/VACNTs further give the proof of the even distribution of MoS2. It can be believed that MoS2 embedded uniformly into VACNTs can effectively avoid aggregation and restacking of MoS2 nanosheets, subsequently ensure the whole structural integrity, eventually lead to good cycling stability (Fig. S2†).
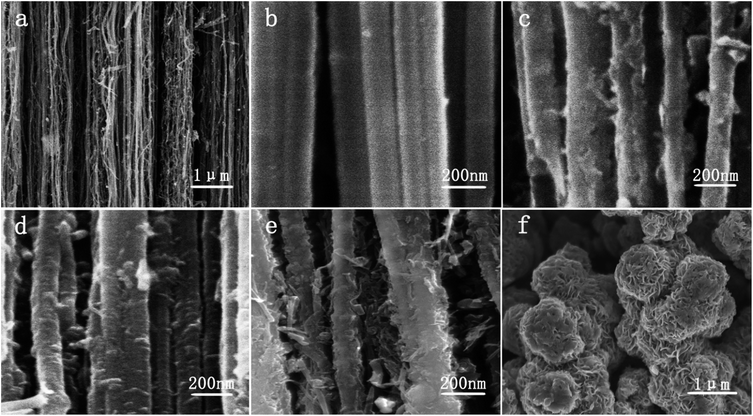 | ||
| Fig. 2 Detailed microstructure characterization from SEM of (a and b) VACNTs, (c) MoS2/VACNTs-1, (d) MoS2/VACNT S-2, (e) MoS2/VACNTs-3 and (f) pristine MoS2. | ||
3.2. Electrochemical performance
TG analysis was carried out from room temperature to 800 °C under air to determine the MoS2 content contained in MoS2/VACNTs composites. As shown in Fig. 4a, these TG curves display one distinct region with weight loss from 400 °C to 550 °C, due to the oxidation of MoS2 to MoO3 as well as combustion of VACNTs.14,30 The content of MoS2 in composites materials can be precisely calculated from this equation: affirming loss content from MoS2 to MoO3 is 40% according to MoS2 TG curve and from VACNTs to CO2 is 100% seen from VACNTs TG curve, one has 4.0314 × 40% + 4.0314(1 − x) = 67.8% × 4.0314. Therefore, x = 0.52. On the basis of accurate calculations, the MoS2 contents of the MoS2/VACNTs-1, MoS2/VACNTs-2 and MoS2/VACNTs-3 are determined to be around 37.15%, 52%, and 59.9%, by weight, respectively.Fig. 4b and c show the representative cyclic voltammograms (CV) of MoS2/VACNTs-2 and pristine MoS2 from the 1st to the 6th cycle and the CV patterns are consistent with previous reports. In the first cathodic sweep, the peak at approximate 1.1 V is attributed to the transformation of MoS2 from trigonal prisms to octahedral phases for lithium storage in the MoS2/VACNTs composites.24 Besides, the peak at 0.5 V can be attributed to the conversion reaction of MoS2 to Li2S.24 Remarkably, these two reduction peaks disappear in subsequent reduction cycles. Instead, two new produced peaks at 2.0 V and 1.2 V might correspond to the insertion of Li+ ions into the MoS2 nanosheets and the conversion reaction shown above, respectively. Such a modification in CV curve shape is common to electrode materials based on the conversion reaction mechanism and reflects permanent structural change during the first cycle. In the first six anodic sweeps, there is a pronounced oxidation peak centered at 2.3 V in Fig. 4b, which is typically attributed to the oxidation of Li2S to S, while a series of broad and weak oxidation peaks ranging from 1.4 V to 1.8 V should be attributed to the delithiation of LiXMoS2, moreover, peaks at about 0.1 V reveal the reversible lithium storage in VACNTs. Fortunately, CV curves for subsequent cycles almost overlap, which indicates excellent structural stability of these MoS2/VACNTs during the charge/discharge process compared with pristine MoS2 seen from Fig. 4c exhibiting some irreversible decay. In the first discharge process as shown in Fig. 4c, the peaks at 1.5 V and 0.5 V are attributed to MoS2 phase change for lithium storage and conversion of MoS2 to Li2S and molybdenum metal, respectively.5 In the first charge process, the doublet centered at 1.6 V as well as a large broad peak at 2.3 V are attributed to delithiation of LiXMoS2 and the formation of sulphur.5 Besides, in the subsequent cycling the dominant cathodic peak at around 0.5 V gets weaker indicating the consumption of the active materials, i.e. LiXMoS2 and MoS2.5
The rate performances of all the materials were evaluated at discharge rates from 100 to 2000 mA g−1, as shown in Fig. 4d. From the rates profiles, an improved rate property of MoS2/VACNTs-2 can be clearly seen. The reversible capacity of MoS2/VACNTs-2 is stable at about 1078 mA h g−1 after 1st cycle at the current density of 100 mA g−1. As the discharge rates increase, the MoS2/VACNTs-2 shows the highest discharge capacity at any current density: 997 mA h g−1 at 200 mA g−1, 927 mA h g−1 at 500 mA g−1, 864 mA h g−1 at 1000 mA g−1 and 789 mA h g−1 at 2000 mA g−1 sustaining over 95% coulombic efficiency during each cycling. In contrast, MoS2/VACNTs-1, MoS2/VACNTs-3 and pure MoS2 nanosheets retained capacities of only 532, 677 and 288 mA h g−1 at 2000 mA g−1 with capacities of 1762, 1429 and 1367 mA h g−1 for the first cycle, respectively. Moreover, this improved rate performance of MoS2/VACNTs-2 can be observed from charge–discharge voltage curves in Fig. 4e.
Fig. 4f shows the electrochemical impedance spectra (EIS) responses for all of the composites. It can be seen that the impedance spectra of all the electrodes consists of a semicircle in the high-medium frequency region and a slanted line in the low frequency region. The semicircle is related to the charge transfer resistance (Rct) at the electrode/electrolyte interface and the linear represents the diffusion impedance of lithium ions in the MoS2.18,30 As shown in Fig. 4f, the MoS2/VACNTs composites exhibit significant reduction of the charge transfer resistance in comparison to the pristine MoS2, indicating that VACNTs could indeed improve the kinetics of the MoS2. In addition, compared to the MoS2/VACNTs-2 and MoS2/VACNTs-3, the semicircle of MoS2/VACNTs-1 is smaller. The possible reason is that the increased content of VACNTs in composite would make the transport pathways for electron and Li+ to the MoS2 particles become more complicated, hence, exhibits a relatively improved conductivity.
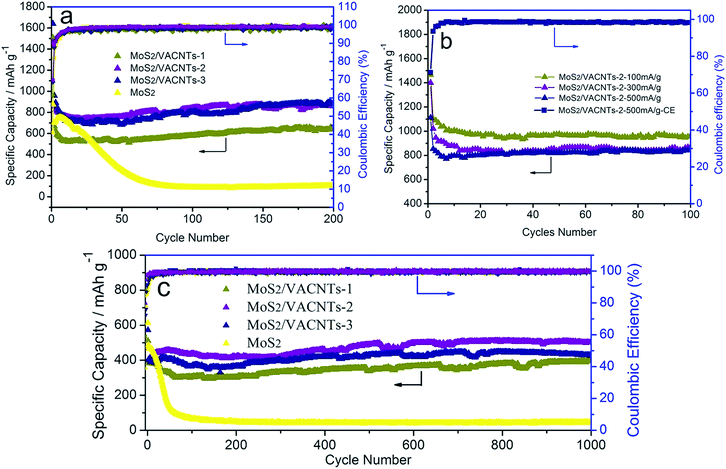
Fig. 5a and c show cycling performance of MoS2/VACNTs-1, MoS2/VACNTs-2, MoS2/VACNTs-3 and pristine MoS2 at 1000 mA g−1 and 5000 mA g−1, respectively. It can be seen that MoS2/VACNTs composites possess excellent cycle stability compared to pristine MoS2 that synthesized by hydrothermal process, for the capacities of MoS2/VACNTs remains unchanged after first cycle while pristine MoS2 shows negligible capacity after 60 cycles. Moreover, MoS2/VACNTs-2 exhibits better electrochemical properties retaining capacities of 817 mA h g−1 at 1000 mA g−1 and 512 mA h g−1 at 5000 mA g−1 with capacities of 1624 and 478 mA h g−1 for the first cycle and above 95% coulombic efficiency after the first five cycles, while MoS2/VACNTs-1 and MoS2/VACNTs-3 retain capacities of 579, 793 mA h g−1 at 1000 mA g−1 and 397, 407 mA h g−1 at 5000 mA g−1 with capacities of 1494, 1449 and 507, 574 mA h g−1 for the first cycle, respectively. The MoS2/VACNTs-2 electrode also displays excellent cycling stability even at other current density (100, 300 and 500 mA g−1), as illustrated in Fig. 5b. The discharge capacity of the MoS2/VACNTs-2 achieved capacity of about 969, 868 and 815 mA h g−1 after 100 cycles at 100, 300 and 500 mA g−1, respectively. In addition, the corresponding coulombic efficiency of MoS2/VACNTs-2 at 500 mA g−1 rapidly rises from 71% to 99% after five cycles.
Above results have demonstrated that MoS2/VACNTs-2 showed improved properties towards the storage of Li+. The improvement of the electrochemical performance, especially the outstanding cycling stability, could be attributed to following reasons based on their microstructure. Firstly, highly conductive 3D VACNTs with well-defined regular pore structure can provide 1D electron-transport pathways that will facilitate the electronic and Li+ diffusion rate, and decrease the polarization at a large current density. Secondly, the VACNTs with appropriate inter-tube space serve as buffer matrix to accommodate the volume expansion/contraction of MoS2 and stabilize the electrode structure during the lithiation/delithiation process.
4 Conclusions
This work sheds light-sight on the excellent cyclic stability anodes for LIBs associated with MoS2 active substance. It is demonstrated effective way to use VACNTs as conductive additives to improve electrical performance in spite of other MoS2/C composites. To synthesize this MoS2/VACNTs composite, we have adopted a simple hydrothermal method with the nebulization-assisted infiltration. So-obtained composites possess original single-direction-oriented hollow based 3D arrays which plays a dominant role in their excellent electrochemical properties. Moreover, these excellent properties in LIBs anodes also take the advantages of synergistic effects with MoS2 and VACNTs in terms of high theoretical capacity from MoS2 and high electronic conductivity from VACNTs, respectively. Here, we present detailed and critical analyses of their electrical properties. Reversible capacities of 1078 mA h g−1 at 100 mA g−1 have achieved for these MoS2/VACNTs composites. Furthermore, an impressive capacities of 789 mA h g−1 at 2000 mA g−1 was exhibited for their good rate capability. Mostly important, such-obtained MoS2/VNCATs composites exhibits extremely-excellent cycling stability, for the MoS2/VACNTs electrode can still deliver a discharge capacity of 512 mA h g−1 after 1000 cycles at 5000 mA g−1, compared with pristine MoS2 (negligible discharge capacity at the 70th cycle). Thus it can be see, this work greatly improves the prevailing problem of capacity attenuation and the intimate contact of MoS2 and VACNTs can effectively avoid the aggregation and restacking of MoS2 during the lithium insertion and extraction process, thus remarkably enhancing the structural integrity of electrode, apart from promising lithium-ion battery anode, the MoS2/VACNTs composite also has immense potential for applications in other areas such as supercapacitor, catalysis, and sensors.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51272073, 51572078 and 51541203) and the Scientific Research Fund of Hunan Province (2015JJ2033).Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed
.
- J. Y. Li, Y. Hou, X. F. Gao, D. S. Guan, Y. Y. Xie, J. H. Chen and C. Yuan, Nano Energy, 2015, 16, 10–18 CrossRef CAS
.
- X. Q. Xiong, W. Luo, X. L. Hu, C. J. Chen, L. Qie, D. F. Hou and Y. H. Huang, Sci. Rep., 2014, 5, 9254 CrossRef PubMed
.
- F. Lin, I. M. Markus, D. Nordlund, T. C. Weng, M. D. Asta, H. L. Xin and M. M. Doeff, Nature, 2014, 5, 3529–3537 Search PubMed
.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Energy Environ. Sci., 2014, 7, 209–231 CAS
.
- I. Jeon, D. Kutsuzawa, Y. Hashimoto, T. Yanase, T. Nagahama, T. Shimada and Y. Matsuo, Org. Electron., 2015, 17, 275–280 CrossRef CAS
.
- M. Shanmugam, T. Bansal, C. W. Durcan and B. Yu, Appl. Phys. Lett., 2012, 100, 153901 CrossRef
.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nature, 2012, 11, 963–969 CrossRef CAS PubMed
.
- L. P. Hansen, Q. M. Ramasse, C. Kisielowski, M. Brorson, E. Johnson, H. Topsøe and S. Helveg, Angew. Chem., Int. Ed., 2011, 50, 10153 CrossRef CAS PubMed
.
- C. Yang, Z. X. Chen, I. Shakir, Y. Xu and H. B. Lu, Nano Res., 2016, 9(4), 951–962 CrossRef CAS
.
- D. W. He, Y. H. Zhang, Q. S. Wu, R. Xu, H. Y. Nan, J. F. Liu, J. J. Yao, Z. L. Wang, S. J. Yuan, Y. Li, Y. Shi, J. L. Wang, Z. H. Ni, L. He, F. Miao, F. Q. Song, H. X. Xu, K. Watanabe, T. Taniguchi, J. B. Xu and X. R. Wang, Nat. Commun., 2014, 5, 5162–5169 CrossRef CAS PubMed
.
- J. Xiao, X. J. Wang, X. Q. Yang, S. D. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21, 2840–2846 CrossRef CAS
.
- L. David, R. Bhandavat, U. Barrera and G. Singh, Sci. Rep., 2015, 5, 9792 CrossRef CAS PubMed
.
- X. Xu, Z. Y. Fan, X. Y. Yu, S. J. Ding, D. M. Yu and X. W. Lou, Adv. Energy Mater., 2014, 1400902 CrossRef
.
- J. W. Zhou, J. Qin, X. Zhang, C. S. Shi, E. Z. Liu, J. J. Li, N. Q. Zhao and C. N. He, Nano, 2015, 9(4), 3837–3848 CAS
.
- A. V. Murugan, M. Quintin, M. Delville, G. Campet, C. S. Gopinath and K. Vijayamohanan, J. Power Sources, 2006, 156, 615–619 CrossRef CAS
.
- K. Chang and W. X. Chen, Chem. Commun., 2011, 47, 4252–4254 RSC
.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10(4), 1–6 CrossRef PubMed
.
- Z. H. Yu, Y. M. Pan, Y. T. Shen, Z. L. Wang, Z. Y. Ong, T. Xu, R. Xin, L. J. Pan, B. G. Wang, L. T. Sun, J. L. Wang, G. Zhang, Y. W. Zhang, Y. Shi and X. R. Wang, Nat. Commun., 2014, 5, 5290–5296 CrossRef CAS PubMed
.
- P. P. Wang, H. Y. Sun, Y. J. Ji, W. H. Li and X. Wang, Adv. Mater., 2014, 26, 964–969 CrossRef CAS PubMed
.
- X. Q. Xie, Z. M. Ao, D. W. Su, J. Q. Zhang and G. X. Wang, Adv. Funct. Mater., 2015, 25, 1393–1403 CrossRef CAS
.
- X. H. Cao, Y. M. Shi, W. H. Shi, X. H. Rui, Q. Y. Yan, J. Kong and H. Zhang, Small, 2013, 9, 3433–3438 CrossRef CAS PubMed
.
- K. Chang and W. X. Chen, Nano, 2011, 5(6), 4720–4728 CAS
.
- S. J. Ding, J. S. Chen and X. W. Lou, Chem.–Eur. J., 2011, 17, 13142–13145 CrossRef CAS PubMed
.
- H. J. Yoo, A. P. Tiwari, J. T. Lee, D. Y. Kim, J. H. Park and H. Lee, Nanoscale, 2015, 7, 3404–3409 RSC
.
- Y. M. Shi, Y. Wang, J. I. Wong, A. Y. S. Tan, C. L. Hsu, L. J. Li, Y. C. Lu and H. Y. Yang, Sci. Rep., 2013, 3, 2169 Search PubMed
.
- E. Frackowiak and F. Beguin, Carbon, 2002, 40, 1775–1787 CrossRef CAS
.
- G. L. Che, B. B. Lakshmi, E. R. Fisher and C. R. Martin, Nature, 1998, 393, 346–349 CrossRef CAS
.
- S. K. Das, Mater. Lett., 2014, 130, 240–244 CrossRef CAS
.
- Z. Yang, X. M. Zhou, Z. P. Jin, Z. Liu, H. G. Nie, X. A. Chen and S. M. Huang, Adv. Mater., 2014, 26, 3156–3161 CrossRef CAS PubMed
.
- W. N. Deng, X. H. Chen, Z. Liu, A. P. Hu, Q. L. Tang, Z. Li and Y. N. Xiong, J. Power Sources, 2015, 277, 131–138 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17042k |
| This journal is © The Royal Society of Chemistry 2016 |