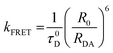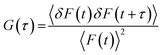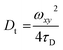DOI:
10.1039/C6RA14804B
(Paper)
RSC Adv., 2016,
6, 71989-71998
Effect of the submicellar concentration of bile salts on structural alterations of β-casein micelles†
Received
7th June 2016
, Accepted 18th July 2016
First published on 19th July 2016
Abstract
The protein self-assembly behavior of β-casein micelles (β-CMs) is investigated in the presence of two bile salts namely sodium deoxycholate (NaDC) and sodium cholate (NaCh) in an aqueous phosphate buffer solution at pH 7.4. The structural behavior and self-assembling properties of β-CMs in the presence of a submicellar concentration of NaDC and NaCh were studied by steady-state and time-resolved fluorescence spectroscopy, fluorescence correlation spectroscopy (FCS) and dynamic light scattering (DLS). The change of critical micellar concentration (CMC) of β-CMs in the presence of NaDC and NaCh is determined taking pyrene as a fluorescence probe. We carried out fluorescence resonance energy transfer (FRET) studies employing coumarin-153 (C-153) as a donor and rhodamine 6G (R6G), as an acceptor in order to probe the structural and dynamic nature of the aggregates. Through Fluorescence Correlation Spectroscopy (FCS) the translational diffusion of R6G in β-CMs and the presence of NaDC and NaCh provide the structural dynamics of these systems. The results are quite comparable to the hypothesis that bile salts affect protein self-assembly through both changes in aqueous structure as well as employing hydrophobic interactions. All the experimental studies support that NaDC forms larger aggregates through complexation with β-CMs than NaCh because of the more hydrophobic character of NaDC compared to NaCh.
1. Introduction
Biopolymers and naturally occurring biosurfactants are widely used in the food, pharmaceutical, agriculture, and cosmetics industries.1–2 Therefore, their molecular level interactions are quite important to understand modifying the final properties of products which are manufactured. Thorough research on intermolecular interactions between biopolymers (proteins or polysaccharides) and bio-surfactants (bile salts or Tween 20) provides many new perceptions of the processes and mechanistic details of many reactions occurring in biological systems. There is very little literature documentation on proteins and bile salts interaction.3–6 The use of bile salts in the purification of proteins involves several steps, like selective solubilization of membranes, chromatographic separation, and reconstitution of proteins.7–9 There are still a large number of opportunities in this major field to study the protein–bile salt interaction. We selected bile salts as biosurfactant to study its effects on self-assembly and we demonstrated it through micellization of β-casein protein.
Bovine milk constitutes of four main caseins among which one is β-casein.10,11 Among the four principal caseins, β-casein is the most amphiphilic constituent in the bovine milk.12–17 Categorically β-casein is a phosphoprotein sensitive to calcium which consists of a polypeptide chain having molecular weight 24 kDa and also containing 209 amino acids. Its isoelectric point (pI) is 4.5.18 β-Casein is categorized as an “intrinsically unstructured/disordered” protein. A distinctive amino acid composition is connected to the intrinsically unstructured/disordered regions, which is characterized by a high content of proline.19,20 However, β-casein self-assembles to form nanosized micellar structures which are stable at neutral pH and this is quite comparable to the same obtained from amphiphilic block-copolymers and surfactants.21 β-Casein is proposed as an potential natural substitute of synthetic block copolymers for the construction of an oral drug delivery system.22,23
Bile salts are important naturally occurring amphiphilic molecules. It is well studied that these amphiphilic molecules are composed of a hydrophobic surface and a hydrophilic surface in their nonplanar steroidal skeleton.24–25 They have drawn a great deal of interest since they have wide applications in various biological and pharmaceutical systems. Cholesterol is converted into the above mentioned bioactive molecules in the liver and they act a very crucial role in living organisms where they solubilize the lipids.26–28 Because of their amphiphilic nature bile salts quite easily and spontaneously form aggregates in the aqueous solution. Again the reported complex aggregation behavior of the aggregates is due to significant polydispersity of the micellar solution of bile salt with respect to size and structure of the aggregates.29–32 Two types of aggregates, primary and secondary aggregates exist with changing the bile salts concentration.26 The most important bile salts studied for biological model systems include cholates and deoxycholates. The CMC (critical micellar concentration) of bile salts are well reported where for NaDC it varies from 4–6 mM and in case of NaCh it ranging from 12–16 mM.33 In living organisms bile salts help to solubilize excess lipids, fat soluble vitamins and cholesterol when the concentrations are quite high (≥CMC).34,35 However, when the concentrations are sufficiently low (<CMC (submicellar)) they effectively attach with the membranes and as a result the intervesicular phospholipid transfer process rate is enhanced.36 Submicellar concentration of bile salt on lipid bi-layer is well studied and it is demonstrated that at this concentration, bile salts are more effective for the interaction with lipid bi-layer.36–38
It is possible to alter the characteristics of amphiphilic systems and their self-assembly behaviors by simply mixing them. The micellar properties of different surfactants (Triton X-100, Tween 80 and CTAB etc.) in combination with bile salts have been explored using various spectroscopic techniques.39–41 In the last decades, a significant number of investigations have been performed to analyze the self-assembly behaviour of β-CMs with different types of additives (sugar, chitosan etc.).21,42–46 Guo et al. studied the interaction of β-CMs with various types of imidazolium based ionic liquids.42,44 However there is no study based on the interaction of bile salts and β-CMs. A large number of different ultrafast fluorescence techniques e.g. photoinduced electron transfer, solvation, rotational dynamics, FRET, and FCS have been used to make out the structural and conformational dynamics of different macromolecules.40,47,48 Among them FRET and FCS are powerful techniques to characterize the structural dynamics of a wide number of nanoscaled organized systems like micelles, mixed micelles, vesicles, reverse micelles, and microemulsions.40,47–49 The FRET signal is only a manifestation of the separation distance between the donor and acceptor and this is quite useful to present a concept regarding the structural nature of a self-assembled organized system. In recent literature, FCS has been extensively applied to study the diffusion coefficient of a molecule in simple solutions as well as in various organized assemblies at the single molecular level.40,48,49 For that purpose we have used FCS which successfully gives insight into the organization and dynamical properties of the self-assemblies.49
In this particular study, we examine the interaction of β-CMs with submicellar concentration of bile salts. NaDC and NaCh were used as representative bile salts to study the self-assembly behavior of β-CMs solutions at phosphate buffer of 10 mM at pH 7.4. The self organization of the as-described linear unstructured protein β-casein, with these bile salts was studied using pyrene fluorescence measurement. There is no report on the self-assembly behavior of β-CMs in presence of bile salts. We also have studied FRET involving C-153 (donor) and R6G (acceptor) in order to develop an idea regarding the microenvironment formed by the donor and acceptor molecules which are implanted in self-organized nanostructures having significant biological importance. We used FCS for the study of the translational diffusion of R6G at a single molecular level in β-CM and its mixture combination with these bile salts.
2. Experimental section
2.1. Materials and method
We received coumarin 153 (C-153, laser grade) and rhodamine 6G (R6G, laser grade) from Exciton and used as received. We purchased pyrene, β-casein from bovine milk (Bioultra 98%, PAGE) from Sigma-Aldrich. The bile salts (NaDC and NaCh) were also brought from Sigma Aldrich and used without further purification. Submicellar concentration of NaDC and NaCh (1 mM) is used in every experiment. We kept the β-casein concentration fixed at 3.0 mg mL−1 which is above the CMC (2.0 mg mL−1) of β-casein42 under the experimental condition. All the required solutions were prepared using phosphate buffer (pH 7.4, 10 mM). A buffer functions to resist changes in hydrogen ion concentration as a result of internal and environmental factors. In addition our experiments were done at pH 7.4 to mimic biological conditions. Regarding the CMC study of pyrene fluorescence measurement at first bile salts were taken then the β-casein were added to the solution and for the rest of the measurements first β-CMs are taken then bile salts were added to the required solution. The structures of all the chemicals used are shown in Scheme 1. The temperature was kept 298 K for all experiments.
 |
| | Scheme 1 Chemical structures of bile salts (NaDC and NaCh), coumarin 153 and rhodamine 6G. | |
2.2. Dynamic light scattering (DLS) measurement
In our present work, Malvern Nano ZS instrument was utilized to record dynamic light scattering (DLS) measurements. This instrument involved a 4 mW He–Ne laser (λ = 632.8 nm) and equipped with a thermostatic sample chamber. In the present instrument, we fixed the detector angle at 173° and used this instrument for DLS measurement. We carried out all the measurements at 298 K.
2.3. Steady state and time-resolved fluorescence measurements
In our particular study, we collected all the absorption spectra employing the Shimadzu (model no UV-2450) spectrophotometer. The steady state fluorescence spectra were obtained using Spex-fluorolog-3 (model FL3-11) and Hitachi (model number F 7000) spectrofluorimeters. For the steady state experiments, all the samples were excited at 408 nm and 336 nm for C-153 and pyrene, respectively. We collected the time-resolved emission decays using time correlated single photon counting (TCSPC) picosecond spectrometer. We have described the details of experimental setup in our earlier reports.40 In a short, picosecond diode laser at 408 nm (IBH, UK, Nanoled) were used as light sources and the signal was detected at a magic angle (54.7°) polarization using Hamamatsu MCP PMT (3809U). In our present working system the typical instrument response function is ∼100 ps. The decays were analyzed using IBH DAS-6 decay analysis software.
The quantum yield of the donor molecules can be calculated by using the equation given below as:
| |  | (1) |
here,
Φ gives quantum yield, Abs represents absorbance,
A gives area under the fluorescence curve, and
n is refractive index of the medium. The subscripts S and R denote the corresponding parameters for the sample and reference, respectively. Coumarin 153 in acetonitrile was taken as reference standard
50 and the quantum yield of the reference standard is,
Φ = 0.56.
2.4. Calculation of FRET parameters
Taking the Förster theory into account we can represent the expression for the rate of fluorescence resonance energy transfer (kFRET) using the following expression:51| | 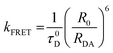 | (2) |
where τ0D represents the lifetime of the donor in the absence of acceptor. RDA is the distance between the molecular centres of the donor and acceptor and at a distance R0 where the efficiency of energy transfer is supposed to be 50%. R0 is defined as Förster distance which is calculated using the following equation:| |  | (3) |
where n represents the refractive index of the medium, QD is the quantum yield of the donor in the absence of acceptor, κ2 is the orientation factor, and J(λ) corresponds to the spectral overlap between the emission spectrum of the donor and the absorption spectrum of acceptor. The relation of J(λ) to the normalized fluorescence intensity of the donor in the absence of acceptor (FD(λ)) and the extinction coefficient of the acceptor (εA(λ)) can be expressed as follows:| |  | (4) |
Generally the value of κ2 is considered as 2/3 for the random orientation of transition dipoles. However, the calculated Förster distance is observed to vary slightly in the whole range of values of κ2. Therefore in the present system we have used κ2 = 2/3 for the calculation of Förster distance.
2.5. Fluorescence correlation spectroscopy (FCS) measurement
In FCS, laser and confocal microscopy are used to produce a very small observation volume (in the order of femoliters (fL)) inside a sample. The diffusion of fluorescent molecules in and out of that volume leads to fluctuations in the fluorescence intensity which can be time-correlated to get a normalized autocorrelation function G(τ):52,53| | 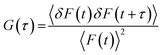 | (5) |
where, 〈F(t)〉 represents the average fluorescence intensity, and δF(t) and δF(t + τ) give the amount of fluctuation in intensity around the mean value at time t and t + τ and these can be expressed as shown in the following equations:| | | δF(t + τ) = F(t + τ) − 〈F(t)〉 | (7) |
In case of a single-component system where diffusion takes place in only three dimensions in the solution phase, we can obtain the diffusion time (τD) by fitting G(τ), the correlation function, using the following equation.53
| |  | (8) |
here,
N gives the number of particles in the observation volume and
ω =
ωz/
ωxy and this is the depth-to-diameter ratio of 3D Gaussian volume. Again if the situation is like that, the diffusing species undergoes an association chemical reaction or change in conformation, which modulate its fluorescence intensity, with a relaxation time (
τR). The correlation function can be written as
49,52| |  | (9) |
here,
A represents the amplitude of the process defined by
τR. The diffusion coefficient of the molecule can be calculated from the diffusion time (
τD) and radius of the observation volume (
ωxy) using the following equation.
| | 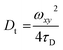 | (10) |
Now the calibration of the structural parameter (ω) of the excitation volume was performed using a sample having known diffusion coefficient [Rhodamine 6G (R6G) in water, Dt = 4.14 × 10−6 cm2 s−1]49,53 by the given equation. The fitted plot is shown in ESI (Fig. S1†).
| |  | (11) |
In the fitting analysis ωxy and ω were kept as free global parameters. From the obtained ω value, Veff is calculated from eqn (12).
| |  | (12) |
Now, we can get the estimated observation volume which is 1.7 fL, with a transverse radius of 283 nm from the universal analysis of the fluorescence correlation function of R6G of varying concentration.
3. Results and discussions
3.1. Pyrene (Py) fluorescence
To determine protein micellization pyrene (Py) is extensively used as a fluorescence probe to understand the polarity within assembled media.51 We can interpret the polarity scale of pyrene in terms of the ratio II/IIII where the II and IIII bands come at 373 nm and 384 nm respectively and that could be used as a quantitative determination of its surroundings polarity. Pyrene II/IIII changes dramatically with the change in the polarity of the medium; the value of II/IIII is higher when the probe molecules are in more polar environment. In polar environment the fluorescence intensity of first vibronic band (II) is enhanced whereas that of the third vibronic band (IIII) remains unresponsive to the change in the surroundings. As a result the plot of the ratio II/IIII contains an inflection point at the CMC when pyrene encounters from hydrophilic to hydrophobic environment of the micelle. The sufficiently lower II/IIII ratio in the post CMC region compared to pre CMC region is assigned because of the preferential partitioning of the nonpolar pyrene into the micellar phase. In Fig. 1 we have shown the change in the CMC of the β-casein micelle in absence and presence of submicellar concentration of NaDC and NaCh. From Fig. 1 it is clear that, with an increasing concentration of both bile salts the sigmoidal curve stretched to the left side. In absence of these bile salts, CMC of casein was ∼2 mg mL−1 which is well reported in previous literature.21,42,43 β-Caseins form micellar structure in such a way that the micellar core constitutes the hydrophobic C-terminal ends and the hydrophilic N-terminal regions spread into the aqueous phase. In presence of NaDC and NaCh, CMC of the protein aggregates decreased to become ∼1.1 mg mL−1 for NaDC and ∼1.5 mg mL−1 for NaCh. Thus it is observed that the decrease of CMC of protein micelle is higher for NaDC than NaCh. The changes which are generated by the bile salts could indicate an increase in the protein propensity to self-associate into micelles. The protein β-casein consists of a large hydrophobic C-terminal domain and a polar negatively charged N-terminal domain. Additionally it can self-assemble into micellar structures containing large hydrophobic regions. Bile salts have a nonplanar steroidal skeleton with a convex surface of hydrophobic groups and a concave surface of hydrophilic groups.26,32 Thus the hydrophobic interaction drives the binding process of bile salts to β-CMs.6,43 Livney et al. reported that the CMC of β-casein lowered after addition of different type of sugars.43 The hydrophobic surface of the sugars played vital role for the interaction with hydrophobic domains of open-structured proteins. The more hydrophobic sugar have more tendencies to interact with hydrophobic domains of the protein, and as a result they interfere with its association and self-assembly. It is well reported that NaDC is more hydrophobic than NaCh due to presence of one less hydroxyl group in NaDC in its backbone.39 Thus there are more hydrophobic interactions between NaDC and β-CMs compared to NaCh with β-CMs.
 |
| | Fig. 1
I
1/I3 values of pyrene as a function of β-casein concentrations without and with NaDC and NaCh at 298 K. | |
3.2. Steady-state and time resolved investigation of FRET process
FRET can successfully provide useful information about the structural and dynamical properties of the macromolecular assemblies.51 In fact, FRET studies have been used for the characterization of several aspects in macromolecule–ligand complexes, including polymer–surfactant and protein–surfactant systems.54–55 In this particular study, we measured steady-state fluorescence emission spectra of donor (C-153) in β-CMs and β-CMs–bile salts complexes. In aqueous buffer, the emission maxima of C-153 were observed to be 550 nm, while for the β-CMs solution, the emission maximum of C-153 was found to be at 533 nm (Table 1). Thus in the emission spectra of C-153, we can observe a significant blue shift in β-CMs solution compared to that in water. This fact implies that the C-153 is encapsulated in the hydrophobic region of the β-CMs. Again in presence of NaDC with β-CMs the emission maximum of C-153 was at 525 nm which was further blue shifted compared to β-CMs. The results indicate that the hydrophobicity of the β-CMs increases in presence of submicellar concentration of NaDC. However in presence of submicellar concentration of NaCh the emission maximum of C-153 was at 530 nm which was at less blue ended region than in case of NaDC. Thus the NaDC interacts with β-CMs in greater extent than NaCh through hydrophobic interaction. The hydrophobic interaction between β-CMs and bile salts may play a crucial role during the binding process of the bile salts to β-CMs. However, the emission maxima of C-153 in high concentration of NaDC (greater than the submicellar concentration) with β-CMs is almost same that in only β-CMs. Generally bile salts perturb the organized assemblies in submicellar concentration.37 The bile salts–human serum albumin (HSA) interaction was studied by Mukherjee et al.6 and they highlighted that the hydrophobic nature of the bile salt controls the extent of interaction with the protein.
Table 1 Energy transfer parameters for C153–R6G pair in different systems
| Systems |
λ
maxemi (nm) |
J(λ)a (M−1 cm−1 nm4) |
R
0 (Å) |
|
Experimental error ±5%.
|
| β-CMs only |
533 |
4.16 × 1015 |
54.58 |
| β-CMs + NaDC |
525 |
4.94 × 1015 |
59.42 |
| β-CMs + NaCh |
530 |
4.65 × 1015 |
58.62 |
A very good spectral overlap was observed between the emission spectra of donor (C153) and the absorption spectra of acceptor (R6G) in β-CMs and also in presence of NaDC and NaCh which are shown in Fig. S2 (ESI†). Therefore the basis for the occurrence of FRET is successfully fulfilled. The spectral overlap integral, J(λ) have been calculated involving the emission spectrum of the donor (C-153) and the absorption spectrum of the acceptor (R6G) in β-CMs solution and in the presence of NaDC and NaCh. The numerical values of spectral overlap integral, J(λ), for all the systems are provided in Table 1. In case of only β-CMs the J(λ) value was 4.16 × 1015 M−1 cm−1 nm4 whereas it increases to 4.94 × 1015 M−1 cm−1 nm4 in presence of NaDC and 4.65 × 1015 M−1 cm−1 nm4 in presence of NaCh. This increase in the J(λ) originates because of the blue shift in emission maximum from 533 to 525 nm in presence of NaDC and 533 to 530 nm in presence of NaCh. The blue shifted emission maxima of hydrophobic probe C-153 is observed in bile salts–β-CMs complexes compared to only β-CMs. As a result, the C-153 feels more hydrophobic environment of bile salts–β-CMs complexes than only β-CMs. Thus the quantum yields of C-153 in bile salts–β-CMs complexes are higher than only β-CMs. The quantum yield of C-153 in β-CMs, β-CMs–NaDC and β-CMs–NaCh are 0.338, 0.474 and 0.464 respectively. Effective FRET from donor to acceptor gives rise to a drop in the fluorescence intensity of the donor with significant rise in the fluorescence intensity of the acceptor at the excitation wavelength of 408 nm. The fluorescence intensity of the acceptor (R6G) in presence of donor (C153) increases at the excitation wavelength of 408 nm which also supports the successful FRET phenomenon. FRET efficiency is a relative magnitude so it does not depend on the concentration of donor or acceptor. The changes in the steady-state emission spectra of donor C-153 and in presence of acceptor R6G in β-CMs and in presence of NaDC and NaCh are expressed in Fig. S3 (ESI†). In spite of obtaining much useful information from steady state measurements, we can present further confirmatory results with the help of time-resolved analysis with picosecond setup. In case of time resolved measurements, the FRET processes are governed with changing the donor lifetime with the addition of acceptor. The decrease of lifetime components and their amplitudes of donor as well as in presence of acceptor in β-CMs and in presence of NaDC and NaCh are shown in Table 2. The fluorescence lifetime was recorded at the emission maxima of C-153 in corresponding systems. The decays in which the donor lifetime decreased with the addition of the acceptor are shown in Fig. 2. The average lifetime for bi-exponential fluorescence decay was calculated using the following eqn (13)
here,
τ1 and
τ2 represent the two decay times having contributions
a1 and
a2, respectively. In case of complex situation such as our present work, where there is a distribution of donor–acceptor distances, we have used average lifetime quenching data to calculate energy transfer efficiency, which is implicated to be more precise than the steady-state fluorescence quenching data. We can calculate FRET efficiency (
E) applying the following
eqn (14)| |  | (14) |
where
τDA and
τD are the lifetimes of donor in the presence and absence of acceptor, respectively. The value of highest energy transfer efficiency indicates to the shortest donor–acceptor distance. The energy transfer efficiencies in addition with the other FRET parameters like spectral overlap integral and Förster distances for both the FRET pairs in all the systems have been provided in
Table 1.
Table 2 Picosecond decay parameters of C153 (donor) emission in the presence and absence of R6G (acceptor)
| Systems |
τ
1 (a1) (ns) |
τ
2 (a2) (ns) |
〈τav〉a (ns) |
|
Experimental error ±5%.
|
| C-153 in β-CMs |
1.92 (0.53) |
6.04 (0.47) |
3.86 |
| C-153 + RG in β-CMs |
1.50 (0.62) |
4.93 (0.38) |
2.80 |
| C-153 in casein-NaDC |
1.93 (0.38) |
6.11 (0.62) |
4.52 |
| C-153 + RG in β-CMs–NaDC |
1.48 (0.50) |
5.44 (0.50) |
3.46 |
| C-153 in β-CMs–NaCh |
1.91 (0.51) |
6.00 (0.49) |
3.91 |
| C-153 + RG in β-CMs–NaCh |
1.58 (0.59) |
4.93 (0.41) |
2.95 |
 |
| | Fig. 2 Time-resolved fluorescence decay of C-153 (7 µM) in the absence and presence of R6G (20 µM) in (a) only β-CMs (3 mg mL−1) (b) β-CMs (3 mg mL−1) + 1 mM NaDC and (c) β-CMs (3 mg mL−1) + 1 mM NaCh at 298 K. | |
Now we can determine the distance (r) between the molecular centers of the donor and acceptor employing the energy transfer efficiencies considering the following eqn (15).
| |  | (15) |
In the previous report, it is confirmed that the donor molecules (C-153) were pinpointed solely within the hydrophobic regions, while the acceptor molecules (R6G) were found to reside preferentially into the water-filled interior i.e. hydrophilic regions.47,49 The FRET study explored that the shortest donor–acceptor (D–A) distance for the C-153–R6G pair in only β-CMs systems was 64.14 Å. In presence of NaDC and NaCh it was 72.68 Å and 70.40 Å, respectively. The results reveal that the formation of aggregates with bile salts leads to an enhancement in the D–A distance. This can also be proved by the increased size of the aggregates upon addition of these bile salts to β-CMs. Here also, the measured D–A distance is comparable to the radius of micellar aggregates. From Small Angle Neutron Scattering (SANS) analysis it is shown that β-casein self-associates to ellipsoidal micellar aggregates with the radius of gyration (Rg) 59 Å which is well correlated with our FRET studies.56 The present finding is consistent with the earlier literature reports where it has been shown that with an increase in the size of aggregate rate constant of FRET decreases (Table 3).57 The measured D–A distance can be logically correlated with the thickness of the hydrophobic region by taking the location of the donor and the acceptor molecules into account, provided the FRET occurs within the same system. Thus NaDC form larger hydrophobic region than NaCh through complexation with β-CMs. The resultant effect was displayed in slightly bigger mixed aggregates formation with β-CMs for NaDC compare to NaCh. The new organized assemblies of β-casein micelles with combination of bile salts may be mixed micelles. The hydrophobic amino acid residues which are situating at the coatings of β-CMs can interact with the exposed hydrophobic segments of the bile salts molecules through the hydrophobic interactions. Again hydrophilic part of β-CMs form hydrogen bonding with hydroxyl and carboxylic parts of bile salts forming a strong complex formation between them.
Table 3 Donor–acceptor distances of C-153 and R6G pair in the various systems
| Systems |
Efficiency (E) |
R
DA (Å) |
k
ET (×108 s−1) |
| β-CMs only |
0.27 |
64.14 |
0.98 |
| β-CMs–NaDC |
0.23 |
72.68 |
0.66 |
| β-CMs–NaCh |
0.25 |
70.40 |
0.85 |
3.3. Dynamic light scattering studies
In our present work, we have carried out dynamic light scattering measurements on mixed solution of NaDC and NaCh with β-CMs to obtain additional information on the interaction of β-CMs and bile salts. Fig. 3 and 4 show the distribution of intensities as a function of the apparent hydrodynamic radius in the presence of NaDC and NaCh, respectively. For comparison, we have also included the corresponding plot for the β-CMs alone in Fig. 3 and 4. From the figure the hydrodynamics radius of β-CMs is 22 nm which is reported previously.42 For NaDC–β-CMs systems the PDI was 0.421 and for NaCh–β-CMs systems it was 0.428. The analysis of the mixed systems displays a unimodal distribution of aggregates, which increases slightly in size for the both cases when submicellar concentrations of NaDC and NaCh are added into the systems. Again the unimodal distributions observed in presence of NaDC and NaCh are wider than that of β-CMs. The observed unimodal distribution of mixed β-CMs with NaDC and NaCh implies the newly formed mixed aggregates. This fact could be explained by assuming the system is constituted of the β-CMs–bile salts complexes, with a size slightly greater than that of β-CMs alone. However the size increment of β-CMs in presence of NaDC was higher than that in NaCh which is well correlated with the above FRET study. In case of NaDC–β-CMs complexes it was 31 nm and for NaCh–β-CMs complexes it was 26 nm. Thus DLS study also suggested the larger aggregate formation of β-CMs with NaDC compared to NaCh.
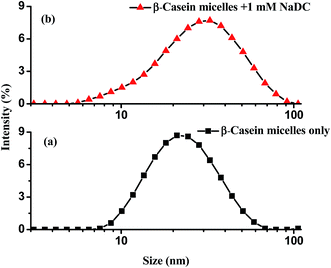 |
| | Fig. 3 Hydrodynamic radii distribution of (a) β-casein micelles only (3 mg mL−1) and (b) β-casein micelle (3 mg mL−1) + 1 mM NaDC at 298 K. | |
 |
| | Fig. 4 Hydrodynamic radii distribution of (a) β-casein micelles only (3 mg mL−1) and (b) β-casein micelles (3 mg mL−1) + 1 mM NaCh at 298 K. | |
3.4. Fluorescence correlation spectroscopy (FCS) studies
FCS technique gives idea regarding the dynamic nature of the organized assemblies. The intensity variations of a fluorophore are observed within a small volume i.e. at the single-molecular level in the FCS technique. The autocorrelation function is produced due to these intensity fluctuations of the fluorophore. Recently, FCS has been extensively used for the study of the diffusion of many fluorophores, chemical kinetics, or the conformational changes of biomolecules in liquid or organized assemblies.58–60 Moreover, using this technique the aggregation of different molecules in aqueous and nonaqueous solvent can also be studied. The diffusion coefficient of dye molecules is dependent on several factors like the size, morphology, and structural heterogeneity of organized assemblies. Therefore, in this particular work, we analyzed the diffusion properties of R6G molecules during the formation of aggregates of different size. At first we have measured the fluorescence autocorrelation trace (FCS trace) of R6G in phosphate buffer at pH 7.4 and then we have measured the FCS trace in β-CMs. To measure the effect of NaDC and NaCh on β-CMs, the FCS traces are collected for the mixture of NaDC and NaCh with β-CMs at pH 7.4. The normalized autocorrelation traces are shown in Fig. 5 and the fitted curves are depicted in Fig. S4 (ESI†). After analyzing the FCS data by the MATLAB software we found the diffusion time of the respective probe molecules from where the values of diffusion coefficients have been calculated. The variation of diffusion times along with diffusion coefficients are shown in Table 4. Now it is possible to understand the nature of the aggregates by using the comparison of the diffusion coefficient (Dt) of the dye molecule in different aggregates. We have fitted the FCS traces in buffer with a Dt value of ∼420 µm2 s−1. The obtained diffusion coefficient value in β-CMs was much less than that of buffer, and it is due to R6G entrapped in β-CMs. In presence of NaDC the Dt value becomes extremely slow with the calculated diffusion coefficients of 76 µm2 s−1 and in presence of NaCh it was 93 µm2 s−1. Thus the slow diffusional motion in presence of NaDC and NaCh compared to only β-CMs could be the dynamic nature of the large aggregates formation by NaDC and NaCh with β-CMs. Furthermore the formation of aggregates is larger in case of NaDC than in presence of NaCh well supported by FCS study.
 |
| | Fig. 5 Normalized autocorrelation curve of R6G (2 nM) in various systems at 298 K. | |
Table 4 Changes in diffusion time and diffusion coefficient of R6G in different systems
| Systems |
Diffusion time (τD, µs) |
Diffusion coefficienta (Dt, µm2 s−1) |
|
Experimental error ±5%.
|
| Buffer |
64 |
420 |
| β-CMs only |
197 |
136 |
| β-CMs–NaDC |
350 |
76 |
| β-CMs–NaCh |
290 |
93 |
4. Conclusion
The interactions between a biological charged diblock copolymer, β-CMs, and biosurfactant, bile salts (NaDC and NaCh) have been studied successfully by steady-state and time-resolved fluorescence spectroscopy, FCS and DLS measurements. The main driving force for the mixed self-assembly formation is the hydrophobic interactions and hydrogen bonding between bile salts and β-CMs. FRET has been effectively functioned among two different D–A pairs as an effective optical tool to understand the formation of microstructure with bile salts and β-CMs. FCS studies also suggested the structural dynamics by measuring the translation diffusion of R6G molecule in the required systems. Overall results reflect that NaDC form larger aggregates than NaCh by complexation with β-CMs due to more hydrophobic nature of NaDC compared to NaCh.
Acknowledgements
N. S. is thankful to SERB, Department of Science and Technology (DST) Government of India, for generous research grant. J. K. is thankful to UGC for research fellowship. A. R. and R. D. are thankful to CSIR for research fellowships.
Notes and references
-
K. Holmberg, B. Jönsson, B. Kronberg and B. Lindman, Surfactant and Polymers in Aqueous Solutions, 2nd edn, Wiley, Chichester, UK, 2002 Search PubMed.
- D. Otzen, Protein-Surfactant Interactions: A Tale of Many States, Biochim. Biophys. Acta, Proteins Proteomics, 2011, 1814, 562–591 Search PubMed.
- T. Saitoh, T. Fukuda, H. Tani, T. Kamidate and H. Watabane, Equilibrium Study on Interactions Between Proteins and Bile-Salt Micelles by Micellar Electrokinetic Chromatography, Anal. Sci., 1996, 12, 569–573 Search PubMed.
- N. C. Robinson and C. Tanford, The Binding of Deoxycholate, Triton X-100, Sodium Dodecyl Sulfate, and Phosphatidylcholine Vesicles to Cytochrome b5, Biochemistry, 1975, 14, 369–378 Search PubMed.
- S. Makino, J. A. Reynolds and C. Tanford, The Binding of Deoxycholate and Triton X-100 to Proteins, J. Biol. Chem., 1973, 248, 4926–4932 Search PubMed.
- N. Ghosh, R. Mondal and S. Mukherjee, Hydrophobicity Is the Governing Factor in the Interaction of Human Serum Albumin with Bile Salts, Langmuir, 2015, 31, 1095–1104 Search PubMed.
- Y. Banno, Y. Yada and Y. Nozawa, Purification and Characterization of Membrane-Bound Phospholipase C Specific for Phosphoinositides from Human Platelets, J. Biol. Chem., 1988, 263, 11459–11465 Search PubMed.
- T. Kamataki, K. Maeda, Y. Yamazoe, T. Nagai and R. Kato, Partial Purification and Characterization of Cytochrome P-450 Responsible for the Occurrence of Sex Difference in Drug Metabolism in the Rat, Biochem. Biophys. Res. Commun., 1981, 103, 1–7 CrossRef CAS PubMed.
- D. L. Foster and R. H. Fillingame, Energy-Transducing H+-ATPase of Escherichia coli. Purification, Reconstitution, and Subunit Composition, J. Biol. Chem., 1979, 254, 8230–8236 CAS.
-
H. E. Swaisgood, in Chemistry of the Caseins, ed. P. F. Fox and P. L. H. McSweeney, Advanced Dairy Chemistry, Elsiver, London, 2003, vol. 1a, pp. 139–201 Search PubMed.
- P. Müller-Buschbaum, R. Gebhardt, S. V. Roth, E. Metwalli and W. Doster, Effect of Calcium Concentration on the Structure of Casein Micelles in Thin Films, Biophys. J., 2007, 93, 960–968 CrossRef PubMed.
- G. P. Berry and L. K. Creamer, The Association of bovine β-Casein, The Importance of the C-Terminal Region, Biochemistry, 1975, 14, 3542–3545 CrossRef CAS PubMed.
- C. R. Vessely, J. F. Carpenter and D. K. Schwartz, Calcium-Induced Changes to the Molecular Conformation and Aggregate Structure of Beta-Casein at the Air–Water Interface, Biomacromolecules, 2005, 6, 3334–3344 CrossRef CAS PubMed.
- L. M. Mikheeva, N. V. Grinberg, V. Y. Grinberg, A. R. Khokhlov and C. G. De Kruif, Thermodynamics of Micellization of Bovine β-Casein Studied by High-Sensitivity Differential Scanning Calorimetry, Langmuir, 2003, 19, 2913–2921 CrossRef CAS.
- O. D. Velev, B. E. Campbell and R. P. Borwankar, Effect of Calcium Ions and Environmental Conditions on the Properties of β-Casein Stabilized Films and Emulsions, Langmuir, 1998, 14, 4122–4130 CrossRef CAS.
- L. Tercinier, A. Ye, S. G. Anema, A. Singh and H. Singh, Interactions of Casein Micelles with Calcium Phosphate Particles, J. Agric. Food Chem., 2014, 62, 5983–5992 CrossRef CAS PubMed.
- M. Vincekovic, M. Curlin and D. Jurasin, Impact of the Cationic Surfactant on the Self-Assembly of Sodium Caseinate, J. Agric. Food Chem., 2014, 62, 8543–8554 CrossRef CAS PubMed.
- A. Hambardzumyan, V. Aguié-Beghin, M. Daoud and R. Douillard, β-Casein and Symmetrical Triblock Copolymer (PEO–PPO–PEO and PPO–PEO–PPO) Surface Properties at the Air–Water Interface, Langmuir, 2004, 20, 756–763 CrossRef CAS PubMed.
- I. Portnaya, E. Ben-Shoshan, U. Cogan, R. Khalfin, D. Fass, O. Ramon and D. Danino, Self-Assembly of Bovine β-Casein below the Isoelectric pH, J. Agric. Food Chem., 2008, 56, 2192–2198 CrossRef CAS PubMed.
- C. Moitzi, I. Portnaya, O. Glatter, O. Ramon and D. Danino, Effect of Temperature on Self-Assembly of Bovine β-Casein above and below Isoelectric pH. Structural Analysis by Cryogenic-Transmission Electron Microscopy and Small-Angle X-Ray Scattering, Langmuir, 2008, 24, 3020–3029 CrossRef CAS PubMed.
- I. Portnaya, R. Khalfin, E. Kesselman, O. Ramon, U. Cogan and D. Danino, Mixed Micellization between Natural and Synthetic Block Copolymers: β-Casein and Lutrol F-127, Phys. Chem. Chem. Phys., 2011, 13, 3153–3160 RSC.
- T. Turovsky, R. Khalfin, S. Kababya, A. Schmidt, Y. Barenholz and D. Danino, Celecoxib Encapsulation in β-Casein Micelles: Structure, Interactions, and Conformation, Langmuir, 2015, 31, 7183–7192 CrossRef CAS PubMed.
- I. Portnaya, U. Cogan, Y. D. Livney, O. Ramon, K. Shimoni, M. Rosenberg and D. Danino, Micellization of Bovine β-Casein Studied by Isothermal Titration Microcalorimetry and Cryogenic Transmission Electron Microscopy, J. Agric. Food Chem., 2006, 54, 5555–5561 CrossRef CAS PubMed.
-
D. M. Small, in The Bile Salts, ed. P. P. Nair and D. Kritchevsky, Plenum Press, New York, 1971, vol. 1, pp. 249–256 Search PubMed.
- C. J. O'Connor and R. G. Wallace, Physico-Chemical Behavior of Bile Salts, Adv. Colloid Interface Sci., 1985, 22, 1–111 CrossRef.
- D. M. Small, S. A. Penkett and D. Chapman, Studies on Simple and Mixed Bile Salt Micelles by Nuclear Magnetic Resonance Spectroscopy, Biochim. Biophys. Acta, 1969, 176, 178–189 CrossRef CAS.
- N. Funasaki, M. Fukuba, T. Kitagawa, M. Nomura, S. Ishikawa, S. Hirota and S. Neya, Two-Dimensional NMR Study on the Structures of Micelles of Sodium Taurocholate, J. Phys. Chem. B, 2004, 108, 438–443 CrossRef CAS.
- L. B. Pártay, M. Sega and P. Jedlovszky, Morphology of Bile Salt Micelles as Studied by Computer Simulation Methods, Langmuir, 2007, 23, 12322–12328 CrossRef PubMed.
- G. Esposito, E. Giglio, N. V. Pavel and A. Zanobi, Size and Shape of Sodium Deoxycholate Micellar Aggregates, J. Phys. Chem., 1987, 91, 356–362 Search PubMed.
- F. Lopez, J. Samseth, K. Mortensen, E. Rosenqvist and J. Rouch, Micro- and Macrostructural Studies of Sodium Deoxycholate Micellar Complexes in Aqueous Solutions, Langmuir, 1996, 12, 6188–6196 CrossRef CAS.
- J. Santhanlakshmi, G. L. Shantha, V. K. Aswal and P. S. Goyal, Small-Angle Neutron Scattering Study of Sodium Cholate and Sodium Deoxycholate Interacting Micelles in Aqueous Medium, Proc.–Indian Acad. Sci., Chem. Sci., 2001, 113, 55–62 CrossRef.
- M. Gomez-Mendoza, M. L. Marin and M. A. Miranda, Dansyl Derivatives of Cholic Acid as Tools to Build Speciation Diagrams for Sodium Cholate Aggregation, J. Phys. Chem. Lett., 2011, 2, 782–785 CrossRef CAS.
- S. Reis, C. G. Moutinho, C. Matosa, B. de Castro, P. Gameiroc and J. L. F. C. Lima, Noninvasive Methods to Determine the Critical Micelle Concentration of Some Bile Acid Salts, Anal. Biochem., 2004, 334, 117–126 CrossRef CAS PubMed.
- M. M. A. Elsayed and G. Cevc, The Vesicle-to-Micelle Transformation of Phospholipid–Cholate Mixed Aggregates: A state of the Art Analysis Including Membrane Curvature Effects, Biochim. Biophys. Acta, 2011, 1808, 140–153 CrossRef CAS PubMed.
- A. Hildebrand, R. Neubert, P. Garidel and A. Blume, Bile Salt Induced Solubilization of Synthetic Phosphatidylcholine Vesicles Studied by Isothermal Titration Calorimetry, Langmuir, 2002, 10, 2836–2847 CrossRef.
- J. W. Nichols, Low concentrations of Bile Salts Increase the Rate of Spontaneous Phospholipid Transfer between Vesicles, Biochemistry, 1986, 25, 4596–4601 CrossRef CAS PubMed.
- M. Mohapatra and A. K. Mishra, Effect of Submicellar Concentrations of Conjugated and Unconjugated Bile Salts on the Lipid Bilayer Membrane, Langmuir, 2011, 27, 13461–13467 CrossRef CAS PubMed.
- M. Mohapatra and A. K. Mishra, Photophysical Behavior of 8-Anilino-1-Naphthalenesulfonate in Vesicles of Pulmonary Surfactant Dipalmitoylphosphatidylcholine (DPPC) and Its Sensitivity toward the Bile Salt–Vesicle Interaction, Langmuir, 2013, 29, 11396–11404 CrossRef CAS PubMed.
- V. Patel, B. Bharatiya, D. Ray, V. K. Aswal and P. Bahadur, Investigations on Microstructural Changes in pH Responsive Mixed Micelles of Triton X-100 and Bile Salt, J. Colloid Interface Sci., 2015, 441, 106–112 CrossRef CAS PubMed.
- A. Roy, N. Kundu, D. Banik, J. Kuchlyan and N. Sarkar, How Does Bile Salt Penetration Affect the Self-assembled Architecture of Pluronic P123 Micelles? – Light Scattering and Spectroscopic Investigations, Phys. Chem. Chem. Phys., 2015, 17, 19977–19990 RSC.
- P. K. Jana and S. P. Moulik, Interaction of Bile Salts with Hexadecyitrimethyiammonium Bromide and Sodium Dodecyl Sulfate, J. Phys. Chem., 1991, 95, 9525–9532 CrossRef CAS.
- Y. Liu, L. Yang and R. Guo, Interaction between β-Casein Micelles and Imidazolium based Ionic Liquid Surfactant, Soft Matter, 2013, 9, 3671–3680 RSC.
- O. Setter and Y. D. Livney, The effect of Sugar Stereochemistry on Protein Self-assembly: The Case of β-Casein Micellization in Different Aldohexose Solutions, Phys. Chem. Chem. Phys., 2015, 17, 3599–3606 RSC.
- Y. Liu, L. Yang, H. Mao and R. Guo, Comparative Studies on the Interaction of [C4Mim]Br, and [C8Mim]Br with β-Casein Micelles, Colloids Surf., A, 2014, 441, 581–588 CrossRef CAS.
- F.-G. Wu, J.-J. Luo and Z.-W. Yu, Unfolding and Refolding Details of Lysozyme in the Presence of β-Casein Micelles, Phys. Chem. Chem. Phys., 2011, 13, 3429–3436 RSC.
- C. F. Narambuena, F. S. Ausar, I. D. Bianco, D. M. Beltramo and E. P. M. Leiva, Aggregation of Casein Micelles by Interactions with Chitosans: A Study by Monte Carlo Simulations, J. Agric. Food Chem., 2005, 53, 459–463 CrossRef CAS PubMed.
- U. Mandal, S. Ghosh, D. K. Das, A. Adhikari, S. Dey and K. Bhattacharyya, Ultrafast Fluorescence Resonance Energy Transfer in a Bile Salt Aggregate: Excitation Wavelength Dependence, J. Chem. Sci., 2008, 120, 15–23 CrossRef CAS.
- D. K. Sasmal, A. K. Mandal, T. Mondal and K. Bhattacharyya, Diffusion of Organic Dyes in Ionic Liquid and Giant Micron Sized Ionic Liquid Mixed Micelle: Fluorescence Correlation Spectroscopy, J. Phys. Chem. B, 2011, 115, 7781–7787 CrossRef CAS PubMed.
- S. Ghosh, A. Roy, D. Banik, N. Kundu, J. Kuchlyan, A. Dhir and N. Sarkar, How Does the Surface Charge of Ionic Surfactant and Cholesterol Forming Vesicles Control Rotational and Translational Motion of Rhodamine 6G Perchlorate (R6G ClO4)?, Langmuir, 2015, 31, 2310–2320 CrossRef CAS PubMed; J. Kuchlyan, N. Kundu, D. Banik, A. Roy and N. Sarkar, Spectroscopy and Fluorescence Lifetime Imaging Microscopy To Probe the Interaction of Bovine Serum Albumin with Graphene Oxide, Langmuir, 2015, 31, 13793–13801 CrossRef PubMed.
- G. I. Jones, W. R. Jackson, C.-Y. Choi and W. R. Bergmark, Solvent Effect on Emission Yield and Lifetime of Coumarin Laser Dyes: Requirements for Rotatory Decay Mechanism, J. Phys. Chem., 1985, 89, 294–300 CrossRef CAS.
-
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd edn, Springer, New York, 2006, ch. 9, 13, 14, and 15 Search PubMed.
- S. Sarkar and K. Chattopadhyay, Studies of Early Events of Folding of a Predominately β-Sheet Protein Using Fluorescence Correlation Spectroscopy and Other Biophysical Methods, Biochemistry, 2014, 53, 1393–1402 CrossRef CAS PubMed.
- R. Yadav, B. Sengupta and P. Sen, Conformational Fluctuation Dynamics of Domain I of Human Serum Albumin in the Course of Chemically and Thermally Induced Unfolding Using Fluorescence Correlation Spectroscopy, J. Phys. Chem. B, 2014, 118, 5428–5438 CrossRef CAS PubMed.
- K. Hayakawa, T. Ohyama, T. Maeda, I. Satake and M. Sato, Enhancement of Energy Transfer between Dye Solubilizates in a Polymer/Surfactant Colloid System, Langmuir, 1988, 4, 481–484 CrossRef CAS.
- J. Chen, F. Zeng, S. Wu, J. Zhao, Q. Chen and Z. Tong, Reversible Fluorescence Modulation Through Energy Transfer with ABC Triblock Copolymer Micelles as scaffolds, Chem. Commun., 2008, 5580–5582 RSC.
- S. Ossowski, A. Jackson, M. Obiols-rabasa, C. Holt, S. Lenton, L. Porcar, M. Paulsson and T. Nylander, Aggregation Behavior of Bovine κ- and β-Casein Studied with Small Angle Neutron Scattering, Light Scattering, and Cryogenic Transmission Electron Microscopy, Langmuir, 2012, 28, 13577–13589 CrossRef CAS PubMed.
- C. C. Ruiz, J. M. Hierrezuelo and J. Aguiar, Physicochemical Studies on the Interaction between N-Decanoyl-N-Methylglucamide and Bovine Serum Albumin, Biomacromolecules, 2007, 8, 2497–2503 CrossRef CAS PubMed.
- U. Haupts, S. Maiti, P. Schwille and W. W. Webb, Dynamics of Fluorescence Fluctuations in Green Fluorescent Protein Observed by Fluorescence Correlation Spectroscopy, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13573–13578 CrossRef CAS.
- A. Benda, M. Benes, V. Marecek, A. Lhotsky, W. T. Hermens and M. Hof, How To Determine Diffusion Coefficients in Planar Phospholipid Systems by Confocal Fluorescence Correlation Spectroscopy, Langmuir, 2003, 19, 4120–4126 CrossRef CAS.
- S. D. Verma, N. Pal, M. K. Singh, H. Shweta, M. F. Khan and S. Sen, Understanding Ligand Interaction with Different Structures of G-Quadruplex DNA: Evidence of Kinetically Controlled Ligand Binding and Binding-Mode Assisted Quadruplex Structure Alteration, Anal. Chem., 2012, 84, 7218–7226 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fitted correlation curve of R6G in water, β-CMs, β-CMs–bile salts mixtures and steady state absorption and fluorescence spectra. See DOI: 10.1039/c6ra14804b |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.