A unique high mechanical strength dialdehyde microfibrillated cellulose/gelatin composite hydrogel with a giant network structure
Xuejing Zheng*,
Qiannan Zhang,
Jie Liu,
Ying Pei and
Keyong Tang*
School of Materials Science and Technology, Zhengzhou University, Zhengzhou, Henan 450000, China. E-mail: x.zheng@zzu.edu.cn; biomaterials@foxmail.com
First published on 25th July 2016
Abstract
Microfibrillated cellulose (MFC) with diameters less than 100 nm and a three-dimensional network structure was produced through high-pressure homogenization. MFC was surface modified by periodate to prepare dialdehyde microfibrillated cellulose (DAMFC). DAMFC/gelatin composite hydrogel was prepared by mixing DAMFC with gelatin solution. A Schiff base was formed through the reaction between the aldehyde groups of DAMFC and amino groups of gelatin; therefore, a giant three-dimensional network structure was formed in the composite hydrogel. Since the nano reinforcing agent DAMFC was covalently bonded to the matrix gelatin, the load could be efficiently transferred through the giant network, therefore, the composite hydrogel presented extremely high mechanical properties. The compression strength of DAMFC/gelatin 25/75 (wt/wt) hydrogel dramatically increased to 1.63 MPa, 41 times that of the pure gelatin. Morphology observation revealed that the pore size of the composite hydrogel could be regulated by the DAMFC oxidation level. The composite scaffold demonstrated a good swelling capacity and could successfully maintain its shape in buffer solution. It should be noted that the giant network is different from the double network or fiber reinforced hydrogel. The present work shows that by forming a giant network structure through chemical crosslinking with the reinforcing agent itself, an extremely high mechanical strength composite hydrogel could be obtained.
Introduction
Soft and wet hydrogels, consisting of three-dimensional cross-linked macromolecules and a large amount of water, possess many unique properties such as low sliding friction, swelling/deswelling and stimuli-responsiveness.1,2 Owing to the similarity between hydrogels and soft tissues in the human body, e.g. cartilage, tendons, ligaments, and muscles, hydrogels have drawn great attention as biomaterials, such as artificial bio-organs and extra-cellular matrices. However, most hydrogels are soft, weak, and brittle. They usually fail at a tensile stress less than sub-MPa. The fracture energies of conventional hydrogels are reported to be about 10 J m−2, much smaller than those of cartilage (∼1000 J m−2).3 Svensson et al. prepared a scaffold using bacterial cellulose (BC) and chemical modified BC as matrix, and compositing with collagen respectively, the compression strength of them not exceed 100 kPa.4 The weak, brittle mechanical characteristic of hydrogel limits the extensive application in many areas.One reason for the lack in mechanical strength of a hydrogel is its solution-like nature and heterogeneous structure, stress always concentrated around the shortest chain when force is applied, leading to a failure of the sample at a very low force. Many efforts have been made to improve the mechanical properties of hydrogels.3–6 For instance, double network hydrogels have been demonstrated to achieve high mechanical strength and high mechanical toughness.7–13 The double network hydrogel consists of two polymer networks with contrasting physical properties: a densely crosslinked strong polyelectrolyte (rigid and brittle skeleton) network in low concentrations and a sparsely crosslinked neutral polymer (soft and ductile substance) networks in high concentration. Extensive experimental and theoretical studies have shown that the toughening of the double network gel is based on a local yielding mechanism. The rigid brittle polyelectrolyte serves as a “sacrificial bond” during fracture.10
Cartilage is a type of connective tissue with a tough, flexible matrix made primarily of collagen and other proteins.14 The poor healing potential of articular cartilage has been a well-known concern in orthopedic surgery. Tissue engineering has emerge from the use of biomaterials which replace small area of damaged tissues to three-dimensional matrices which can act as cell carries as well as signal provides for regeneration.2,15 Scaffolds represent one of the key components for tissue engineering approach.16 Numerous biomaterials have been investigated as scaffolds for cartilage repair, including natural and synthetic materials. Scaffolds formed from proteins are particularly suited for tissue engineering as they comprise the structural basis for extracellular matrices of tissues. Moreover, in protein scaffolds, cell adhesion molecules (integrins) can directly bind to the scaffold material. Fibrous protein (collagens, silks) in particular are attractive, due to their architectural features and impressive mechanical properties. Amongst these, collagen and gelatin are widely used due to their chemical and structural similarity to cartilage.17–20 However, as most natural polymeric materials, gelatin scaffold lacks mechanical properties and shows rapid degradation in vivo. Extensive research has focused on mixtures of gelatin with other biological materials for a composite with improved mechanical properties and stability.
Cellulose-based scaffold is also attractive for cartilage tissue engineering.21 Cellulose is a large, linear-chain polymer with an abundance of hydroxyl groups, good biocompatibility, and unique physicochemical properties. It is known that natural plant fibers have a complex hierarchical structure. A single plant fiber (usually 100–200 μm in width) is actually a bundle of fibrils. One fibril is composed of many microfibrils, and one microfibril is composed of hundreds and thousands of cellulose chains. Natural fibers can be processed in different ways to yield reinforcing elements with different mechanical properties. If the diameter of cellulose fibrils is decreased to submicro- or nanoscale, it shows extraordinary reinforcing effect.22,23 Various nanocellulose such as microfibrillated cellulose,24–26 bacterial cellulose (BC)27–30 and their derivatives31–34 have been investigated. Especially, microfibrillated cellulose (MFC) with large aspect ratio and web-like structure has recently attracted a lot of interest due to its super properties, such as high mechanical properties, high expanded surface area, very high aspect ratio and light weight.23,35 Furthermore, the many free reactive hydroxyl groups exposed at the nanocellulose surface can be employed for various chemical modification and functionality to the material, through chemical derivatization or post-functionalization. Chang et al. proposed the method of using transglutaminase, genipin and EDC as the crosslinking agents to prepare the bacterial cellulose/gelatin composite to enhance its physical properties.36 Gao et al. reported the immobilization of gelatin onto bacterial cellulose without utilization of any crosslinking agent.34
In this paper, we present a method to synthesize a unique high mechanical strength dialdehyde microfibrillated cellulose (DAMFC)/gelatin composite hydrogel in which DAMFC containing network structure acts as both a reinforcing agent and a crosslinking agent. In detail, MFC with fine three-dimensional network structure was prepared by high pressure homogenization. DAMFC retaining the fine network structure was obtained from MFC by surface oxidation by periodate. Blending DAMFC with gelatin, the aldehyde groups of DAMFC reacted with the free amine groups of gelatin through Schiff's base and a giant network was therefore formed. The mechanical properties, morphology, and swelling behavior of the DAMFC/gelatin hydrogel were systematically investigated. The present work shows that, by forming a giant network structure, biomass-based hydrogels with dramatically enhanced mechanical strength could be obtained. The giant network is different from the double network or fiber reinforced hydrogel. This work may provide a new method to prepare high mechanical property hydrogel.
Experimental
Materials
Microcrystalline cellulose (MCC) was supplied by Xufu Tianli Pharmaceutical Dressing Co., China. Gelatin (Bloom 240) was from Aladdin. Other chemical agents were analytically pure.Preparation of microfibrillated cellulose (MFC)
MFC was prepared by high pressure homogenizing method following an alkali pretreatment. In brief, MCC and a 10% NaOH solution were put into a beaker and stirred for 2 h at 60 °C. Then MCC was washed thoroughly with distilled water to remove NaOH. The alkali-pretreated MCC suspensions were homogenized at pressure of 1000 bar for 30 passes with a high-pressure homogenizer (APV2000, SPX Flow technology Rosista GmbH, German). White milky MFC suspension was obtained.Preparation of dialdehyde microfibrillated cellulose (DAMFC)
MFC was surface oxidized into DAMFC by NaIO4 with the weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 3
10, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 5
10, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 7
10, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 9
10, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of NaIO4
10 of NaIO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MFC. Proper amount of NaIO4 was added into MFC suspension. The mixture was stirred for 48 h in absence of light at 25 °C. Then the resulting products were dialyzed (the molecular weight cutoff of dialysis membranes is 8000–14
MFC. Proper amount of NaIO4 was added into MFC suspension. The mixture was stirred for 48 h in absence of light at 25 °C. Then the resulting products were dialyzed (the molecular weight cutoff of dialysis membranes is 8000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against deionized water for 2 days to remove the residual NaIO4. The final DAMFC suspension was stored at 4 °C for further use, and its concentration was determined by a weight method.
000) against deionized water for 2 days to remove the residual NaIO4. The final DAMFC suspension was stored at 4 °C for further use, and its concentration was determined by a weight method.
The oxidation level of DAMFC was expressed by carbonyl group content. The carbonyl group content of DAMFC was determined by copper titration.31,37 Copper number (Cu#) is defined as the number of grams of metallic copper (as Cu2O) resulting from the reduction of CuSO4 by 100.00 g of DAMFC. In brief, 1.500 g DAMFC was added into 5.00 mL CuSO4 solution and 95.00 mL Na2CO3–NaHCO3 buffer solution. Then the mixture was kept at 100 °C for 3 h with slow stirring. The resulting mixture was cooled, filtered with Büchner funnel with suction. The DAMFC was washed with 100 mL 5% Na2CO3 solution and then flooded with 250 mL of hot water at 95 °C. DAMFC along with the filter paper were transferred to a 500 mL beaker, and 25 mL of the phosphomolybdic acid was added. Then the mixture was transferred to a Büchner funnel and washed thoroughly with water. The filtrate was collected and diluted with deionized water, then titrated with 0.05 N KMnO4 until the solution color turned to faint pink. A blank test was also performed following the same procedure for MFC. The average of three tests was recorded. The carbonyl group content was calculated according to eqn (1):
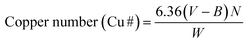 | (1) |
The copper number of each sample was measured in duplicate with an error of less than ±5%. The copper number is an indication of aldehyde groups in fibers. It has been reported38 that there is a linear relationship between the carbonyl group content and copper number shown in eqn (2).
| Carbonyl group content (mmol per 100 g DAMFC) = (Cu# − 0.07)/0.6 | (2) |
Preparation of DAMFC/gelatin composite hydrogel
A mixture was prepared by adding gelatin (5.00 g) into a certain amount of DAMFC suspension (DAMFC/gelatin = 15/85 wt/wt). Keeping DAMFC/gelatin ratio constant, DAMFC (NaIO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MFC = 1
MFC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) was mixed with 5.00 g gelatin with quality ratio of 5/95, 10/90, 15/85, 20/80 and 25/75, respectively. The mixture was kept at 40 °C with slow stirring for 2 h. Ultrasonic vibration was used to remove air bubbles. The mixture was poured into six-well plates at room temperature and kept for 12 h to form hydrogels.
7) was mixed with 5.00 g gelatin with quality ratio of 5/95, 10/90, 15/85, 20/80 and 25/75, respectively. The mixture was kept at 40 °C with slow stirring for 2 h. Ultrasonic vibration was used to remove air bubbles. The mixture was poured into six-well plates at room temperature and kept for 12 h to form hydrogels.
FTIR characterization
MFC, DAMFC, and the lyophilized scaffolds were ground into powder. The samples were mixed with KBr and compressed into tablets. The infrared spectra of the samples were characterized with a Fourier transform infrared analyzer (Thermo Nicolet IR200). 32 scans were signal-averaged with a resolution of 4 cm−1.X-ray diffraction (XRD)
XRD was performed on a PANalytical X'Pert Pro X-ray diffraction (Netherlands) with Cu Kα radiation (λ = 1.54178 Å). The 2θ range was from 3° to 60° in step of 0.033° with a count time of 100 s.Morphology characterization
The samples were quickly frozen using liquid nitrogen and then lyophilized for 24 h. Before SEM observation, all the samples were broken in liquid nitrogen. The fracture surface was sprayed with platinum and observed with a scanning electron microscope (Quanta-200, FEI, USA).Mechanical property measurements
The mechanical properties tests of cylindrical hydrogel samples with a diameter of 22 mm and height of 18 mm were measured using a universal mechanical testing machine, CMT5140. The tests were carried out at a compression speed of 1.5 mm s−1 until the hydrogel broke down. The average of at least five tests was reported.Swelling experiments
Swelling experiments were performed by immersing lyophilized scaffolds in PBS (pH = 7.4) buffer solution at 37 °C until swelling equilibrium. The swelling capacity Me of the composite scaffolds was calculated by eqn (3):| Me (%) = (Wt − W0)/W0 × 100 | (3) |
Results and discussion
Model of giant network structure construction
Fig. 1a shows the schematic that MFC undergo oxidative cleavage in presence of NaIO4 at the C2–C3 glycol bond and resulting in dialdehyde groups. The cross-linking reaction occurs between the aldehyde group in DAMFC and the free ε-amino groups of the lysine and hydroxylysine present in gelatin via the Schiff base as shown in Fig. 1b.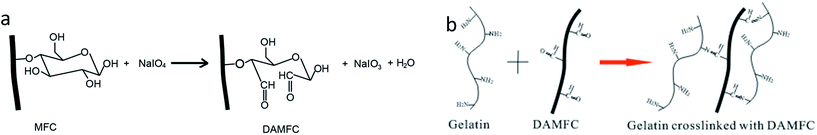 | ||
| Fig. 1 The schematic diagram of the preparation of DAMFC (a) and DAMFC/gelatin composite hydrogel (b). | ||
The model in Fig. 2 illustrates the construction process of the giant network. First, three-dimensional fine network structure is constructed in MFC by high pressure homogenization. Then MFC is oxidized by periodate to prepare DAMFC with abundant aldehyde groups. Since the oxidation takes place in MFC suspension, only the surfaces of MFC microfibrils are oxidized. DAMFC remains the microfibril shape and three-dimensional fine network structure. When DAMFC microfibrils were mixed with gelatin, a Schiff base was formed through the crosslinking reaction between the aldehyde groups of DAMFC and amino groups of gelatin. As a result, a giant network structure forms throughout DAMFC/gelatin composite hydrogel.
Carbonyl group content
Fig. 3 shows the aldehyde content of the DAMFC increased with the weight ratio of NaIO4 to MFC. When the weight ratio of NaIO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MFC is 1
MFC is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the content of aldehyde groups on DAMFC have an observably increase compared with MFC, this maybe the oxidized region was happened on the non-crystalline region initially when the oxidizing agent is not enough. With the NaIO4 concentration increasing, the content of aldehyde groups increased almost linearly. This maybe the non-compact site of the crystalline region was oxidized gradually with the increased of the concentration. After high pressure homogenizing and oxidation process, the DAMFC with high aspect ratio and more reaction groups are apt to form steady network structure and show strength behavior for scaffold applications.
10, the content of aldehyde groups on DAMFC have an observably increase compared with MFC, this maybe the oxidized region was happened on the non-crystalline region initially when the oxidizing agent is not enough. With the NaIO4 concentration increasing, the content of aldehyde groups increased almost linearly. This maybe the non-compact site of the crystalline region was oxidized gradually with the increased of the concentration. After high pressure homogenizing and oxidation process, the DAMFC with high aspect ratio and more reaction groups are apt to form steady network structure and show strength behavior for scaffold applications.
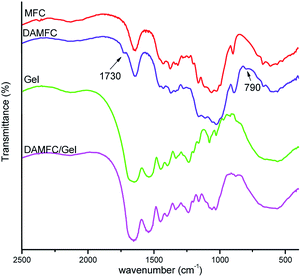
FTIR analysis
Fig. 4 shows the FTIR spectra of MFC, DAMFC, gelatin and DAMFC/gelatin. Compared with MFC, DAMFC exhibited two additional bands around 1730 cm−1 attributing to carbonyl groups and 790 cm−1 corresponding to the bending vibration of –CH of the aldehyde group, suggesting a successful oxidization reaction in DAMFC macromolecular chains. In the spectrum of pure gelatin, we can observe the typical bands such as 1653 cm−1 for the amide I mainly associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, N–H bending vibration at 1543 cm−1 for the amide II and N–H deformation at 1238 cm−1 for the amide III band.39 When gelatin is mixed with DAMFC, a Schiff base is formed between the amino groups of gelatin and aldehyde groups of DAMFC. C
O stretching vibration, N–H bending vibration at 1543 cm−1 for the amide II and N–H deformation at 1238 cm−1 for the amide III band.39 When gelatin is mixed with DAMFC, a Schiff base is formed between the amino groups of gelatin and aldehyde groups of DAMFC. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of Schiff based is in the 1650–1600 cm−1 region, however, this peak is covered by the amide I band of gelatin. The peak at 1730 cm−1 is no longer observed in DAMFC/gelatin spectrum. It indicates that the carbonyl groups of DAMFC have been consumed due to the Schiff base reaction.
N stretching vibration of Schiff based is in the 1650–1600 cm−1 region, however, this peak is covered by the amide I band of gelatin. The peak at 1730 cm−1 is no longer observed in DAMFC/gelatin spectrum. It indicates that the carbonyl groups of DAMFC have been consumed due to the Schiff base reaction.
XRD analysis
X-ray diffraction is a method used generally to evaluate the degree of crystallinity of materials. The XRD characterization of lyophilized hydrogels scaffold was carried out and the results are shown in Fig. 5. In general MFC shows several typical cellulose crystalline peaks in the 2θ range of 10–40°.40 The peak at 14.5° is a composite of the (100) triclinic and the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) monoclinic diffractions. Peak at 16.6° is the composite of (010) triclinic and the (110) monoclinic diffractions. Peak at 20.4° is the composite of (
0) monoclinic diffractions. Peak at 16.6° is the composite of (010) triclinic and the (110) monoclinic diffractions. Peak at 20.4° is the composite of (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2) triclinic and the (012)(102) monoclinic diffractions. Peak at 22.6° is the composite of (110) triclinic and the (200) monoclinic diffractions. Peak at 34.4° is the composite of (
2) triclinic and the (012)(102) monoclinic diffractions. Peak at 22.6° is the composite of (110) triclinic and the (200) monoclinic diffractions. Peak at 34.4° is the composite of (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3)(
3)(![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 4) triclinic and the (023)(004) monoclinic diffractions.41 It can be seen that DAMFC shows diffraction peaks similar to MFC, however, the peak at 20.4° disappears, maybe the result that the oxidation process destroys the cellulose crystalline region, leading to the decrease in relative crystallinity. Moreover, the diffraction peaks of DAMFC are generally weaker than those of MFC, suggesting a decreased crystalline region due to oxidization.32 Pure gelatin exhibits the diffraction peak at 7.8° and 21.8°. MFC/gelatin shows diffraction peaks at 7.8° and 22.6°. While in DAMFC/gelatin, a wide peak seems composed of peaks around 20.2° and 21.8° is observed. It indicates that the Schiff base reaction in DAMFC/gelatin changed the crystalline structure of the sample.
4) triclinic and the (023)(004) monoclinic diffractions.41 It can be seen that DAMFC shows diffraction peaks similar to MFC, however, the peak at 20.4° disappears, maybe the result that the oxidation process destroys the cellulose crystalline region, leading to the decrease in relative crystallinity. Moreover, the diffraction peaks of DAMFC are generally weaker than those of MFC, suggesting a decreased crystalline region due to oxidization.32 Pure gelatin exhibits the diffraction peak at 7.8° and 21.8°. MFC/gelatin shows diffraction peaks at 7.8° and 22.6°. While in DAMFC/gelatin, a wide peak seems composed of peaks around 20.2° and 21.8° is observed. It indicates that the Schiff base reaction in DAMFC/gelatin changed the crystalline structure of the sample.
Morphological characterization
Fig. 6 shows the SEM images of MCC, MFC and DAMFC. It is seen from Fig. 6a that rod-like MCC has width of about 10–30 μm and length of about 40–50 μm. After the high-pressure homogenization, the original rod-like structure of MCC was changed and MFC with uniform and fine microfibrils were successfully obtained. These microfibrils with diameters less than 100 nm do not break during the high-pressure homogenizing but connect with each other and presented a web-like structure (Fig. 6b). Fig. 6c shows the morphology of DAMFC. It is seen that DAMFC still remains the microfibrillar structure and the three-dimensional network structure after oxidation.The hydrogels were lyophilized and the morphology was characterized by SEM. The results are shown in Fig. 7. Three-dimensional porous structures are observed throughout the entire scaffold with pore size ranging from 10 to 50 μm. This type of structure could provide a platform for cell adhesion and proliferation. It is seen that the carbonyl group content of DAMFC exerts obvious influence on the pore size of DAMFC/gelatin composite hydrogels. With the carbonyl group content increases, the average pore size decreases. It indicates that the higher oxidation level results in a higher crosslinking degree between DAMFC and gelatin and therefore a denser network density. The dense giant network leads to a decreased pore size. It is seen that the pore size and pore structure of the composite hydrogels could be regulated by DAMFC oxidation level.
Mechanical property measurements
Compression test were performed to assess the mechanical strength of the hydrogels. The results are shown in Fig. 8 and 9. Fig. 8 is the typical stress–strain curves of the composite hydrogels. Due to the higher mechanical strength and the web-like structure, it is seen that the compression stress of MFC/gelatin hydrogel is enhanced compared to the pure gelatin hydrogel, indicating that the cellulose is helpful to improve the mechanical properties of the hydrogel acting as a reinforcing filler. The addition of DAMFC dramatically increases the stress as well as the strain of the composite hydrogel. Fig. 9 shows the influence of DAMFC content on the compression strength of the composite hydrogel. It is seen that the addition of DAMFC dramatically increases the compression strength of the composite hydrogels. Especially when the content of DAMFC is higher than 10 wt%, a remarkable increase in mechanical strength is presented. For instance, the compression strength of DAMFC/gelatin 25/75 composite hydrogel increases to 1.63 MPa, 41 times of the pure gelatin hydrogel. Fig. 10 shows the compression strength of composite hydrogels with various carbonyl group content of DAMFC. At low oxidation level, the addition of DAMFC does not increase the mechanical strength obviously. However, at higher carbonyl group content, the compression strength increases dramatically. At carbonyl group content of 1.725 mmol per 100 g, the compression strength of DAMFC/gelatin 15/85 is 1.53 MPa, more than 38 times of 0.04 MPa for MFC/gelatin hydrogel. It is seen that the giant network structure formation indeed enhances the mechanical strength of the composite hydrogels. It can be understood that when the aldehyde groups of DAMFC react with the amino groups of gelatin, a giant network forms in the system. DAMFC microfibrils act both as reinforcing agent and crosslinking agent. Since reinforcing agent DAMFC are linked covalently with gelatin macromolecular chains, load can be transferred efficiently throughout the giant network. Therefore, DAMFC microfibrils can bear the stress effectively and give full play as a reinforcing agent. With the content or the carbonyl group content of DAMFC increasing, the amount of aldehyde groups increase and therefore leads to a dense crosslinked giant network in the composite hydrogels. This results in a substantial increase in mechanical strength.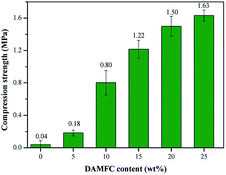 | ||
| Fig. 9 Compression strength of composite hydrogels with different DAMFC content (DAMFC carbonyl group content 1.471 mmol per 100 g). | ||
 | ||
| Fig. 10 Compression strength of composite hydrogels with different DAMFC carbonyl group content (DAMFC content 15 wt%). | ||
Zimmermann et al.42 used MFC to reinforce hydroxypropyl cellulose (HPC). Due to the lack of effective interaction between the reinforcing agent and matrix polymer, the reinforcing effect of MFC hardly displays fully. In their work the modulus of elasticity of HPC could be increased up to 3 times with 20 wt% of MFC. Chen et al.43 reported strong collagen hydrogels by aldehyde-functionalized dextran modification. The resulting collagen/aldehyde-functionalized dextran (Col/DAD) hydrogels are much stronger and show better thermostability than the pristine collagen hydrogel. The maximum compressive strength of the Col/DAD hydrogel is 32.5 ± 1.6 kPa, which is about 20 times more than that of the pristine collagen hydrogel. In our work, the compression strength of DAMFC/gelatin is 41 times of the pure gelatin. It is seen that due to the microfibrillar and three-dimensional structure of DAMFC together with the giant network generated by the chemical crosslinking reaction between the reinforcing agent and matrix, DAMFC/gelatin composite hydrogel shows much higher mechanical strength than similar network in the literature.
Swelling experiments
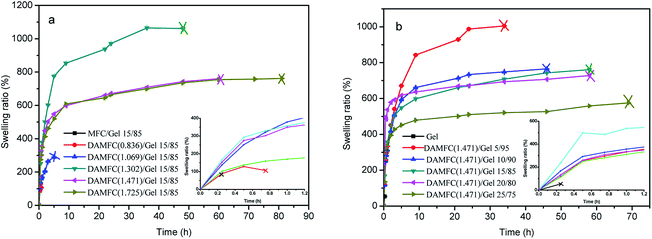
The swelling ability of the scaffold is an important aspect to evaluate its property. It has been indicated that rapid swelling behavior in scaffold is contributed to cells adsorption and growth, and is closely related to the degree of crosslinking. Fig. 11 shows the influence of DAMFC oxidation level and DAMFC content on the swelling behavior of the composite hydrogels. Fig. 11a shows the swelling behavior of DAMFC/gel 15/85 with various carbonyl contents in PBS buffer solution (pH 7.4) at 37 °C. It is seen that MFC/gelatin cannot keep its shape in buffer solution and break down into small pieces quickly in half an hour. At low oxidation level (carbonyl group degree <1.069 mmol per 100 g), DAMFC/gelatin composite scaffold also break into pieces in PBS in less than 10 h (the “×” in figure represents that the sample starts to break in PBS), not available for practical application. Only at higher carbonyl group degree do the scaffolds keep their shape in PBS for more than 50 h, indicating a greatly improved stability in PBS. At carbonyl group content higher than 1.302 mmol per 100 g, the swelling capacity of the scaffold decreases with the carbonyl group content increasing. Fig. 11b shows the influence of DAMFC content on the swelling capacity of the composite scaffold. It can be seen that pure gelatin breaks into pieces quickly in water and could not continue the swelling experiment. In DAMFC/gelatin systems, with the content of DAMFC increase, the swollen scaffolds could maintain their shape for longer time with a lower swelling ratio. This is attributed to the fact that the increase of DAMFC content leads to a higher crosslinking degree and a dense network structure in the system. Consequently, the polymer network has a lower available hydrodynamic free volume to accommodate the water molecules, thereby decrease the matrix swelling. The mobility and relaxation of the polymer chains are hindered and the available free space decreases, which in turn impede the diffusion of water molecules, lowering the swelling ratio. The results in Fig. 11 indicate that it is possible to regulate the swelling behavior of the composites by changing the oxidation level or the content of DAMFC.Conclusions
Dialdehyde microfibrillated cellulose (DAMFC) with three-dimensional network structure can be successfully prepared from MFC by surface oxidation with periodate. Owing to its unique morphology and reactive aldehyde groups, DAMFC can play the role as a reinforcing agent as well as a crosslinking agent. When DAMFC is mixed with gelatin, Schiff base is formed through the reaction between the aldehyde groups of DAMFC and amino groups of gelatin, and therefore endows the composite hydrogel with a giant network structure. Since DAMFC is covalently bonded to gelatin, load could be transferred efficiently in the giant network from matrix gelatin to reinforcing agent cellulose microfibrils, DAMFC/gelatin composite hydrogels showed dramatically enhanced mechanical strength. The composite scaffolds exhibited a three-dimensional network structure and the pore size could be regulated by the oxidation level of DAMFC. The composite scaffolds show a good swelling capacity and could successfully maintain its shape in PBS buffer solution. The present work shows that, by forming a giant network structure through the chemical crosslinking with reinforcing agent itself, an extremely high mechanical strength composite hydrogel would be obtained. The investigated DAMFC/gelatin hydrogel can be a promising candidate in tissue engineering, e.g. cartilage tissue engineering scaffold.Acknowledgements
The financial support of this work by the National Natural Science Foundation of China (Grant No. 51473150 and U1404509) and The Education Department of Henan Province (No. 13A430705 and 17HASTIT009) is gratefully acknowledged.Notes and references
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS PubMed
.
- B. Balakrishnan and R. Banerjee, Chem. Rev., 2011, 111, 4453–4474 CrossRef CAS PubMed
.
- J.-Y. Sun, X. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. Suo, Nature, 2012, 489, 133–136 CrossRef CAS PubMed
.
- A. Svensson, E. Nicklasson, T. Harrah, B. Panilaitis, D. L. Kaplan, M. Brittberg and P. Gatenholm, Biomaterials, 2005, 26(4), 419–431 CrossRef CAS PubMed
.
- K. Haraguchi and T. Takehisa, Adv. Mater., 2002, 14, 1120–1124 CrossRef CAS
.
- K. Haraguchi and H. J. Li, Macromolecules, 2006, 39, 1898–1905 CrossRef CAS
.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS
.
- A. Haque, T. Kurokawa and J. P. Gong, Polymer, 2012, 53, 1805–1822 CrossRef
.
- T. Nakajima, H. Sato, Y. Zhao, S. Kawahara, T. Kurokawa, K. Sugahara and J. P. Gong, Adv. Funct. Mater., 2012, 22, 4426–4432 CrossRef CAS
.
- J. P. Gong, Soft Matter, 2010, 6, 2583–3259 RSC
.
- Y. H. Na, Korea Aust. Rheol. J., 2013, 25(4), 185–196 CrossRef
.
- Q. Chen, H. Chen, L. Zhu and J. Zheng, J. Mater. Chem. B, 2015, 3, 3654–3676 RSC
.
- Z. Li, Y. Su, B. Xie, X. Liu, X. Gao and D. Wang, J. Mater. Chem. B, 2015, 3, 1769–1778 RSC
.
- P. Balasubramanian, M. P. Prabhakaran, M. Sireesha and S. Ramakrishna, in Advances in Polymer Science, ed. T. N. Wassermann, Springer Heidelberg, New York, Dordrecht, London, 2013, p. 173 Search PubMed
.
- L. G. Griffith and G. Naughton, Tissue engineering-current challenges and expanding opportunities, Science, 2002, 295, 1009–1015 CrossRef CAS PubMed
.
- F. J. O'Brien, Mater. Today, 2011, 14, 88–95 CrossRef
.
- Y. Cai, J. Li, C. K. Poh, H. C. Tan, E. S. Thian, J. Y. H. Fun, J. Sun, B. Y. Tay and W. Wang, J. Mater. Chem. B, 2013, 1, 5971–5976 RSC
.
- B. Hoyer, A. Bernhardt, A. Lode, S. Heinemann, J. Sewing, M. Klinger, H. Notbohm and M. Gelinsky, Acta Biomater., 2014, 10, 883–892 CrossRef CAS PubMed
.
- X. Li, Y. Zhao, W. Ding, J. Wei, S. Han, X. Shang, B. Wang, B. Chen, Z. Xiao and J. Dai, ACS Appl. Mater. Interfaces, 2015, 7(25), 13960–13971 CAS
.
- B. Hoyer, A. Bernhardt, S. Heinemann, I. Stachel, M. Meyer and M. Gelinsky, Biomacromolecules, 2012, 13(4), 1059–1066 CrossRef CAS PubMed
.
- F. A. Müller, L. Müller, I. Hofmann, P. Greil, M. M. Wenzel and R. Staudenmaier, Biomaterials, 2006, 27, 3955–3963 CrossRef PubMed
.
- A. K. Bledzki and J. Gassan, Prog. Polym. Sci., 1999, 24, 221–274 CrossRef CAS
.
- X. Zheng, J. Liu, Y. Pei, J. Li and K. Tang, Composites, Part A, 2012, 43, 45–52 CrossRef
.
- I. Siró and D. Plackett, Cellulose, 2010, 17, 459–494 CrossRef
.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC
.
- A. P. Mathew and K. Oksman, Cellulose, 2012, 19, 139–150 CrossRef CAS
.
- Z. Yan, S. Chen, H. Wang, B. Wang, C. Wang and J. Jiang, Carbohydr. Res., 2008, 343, 73–80 CrossRef CAS PubMed
.
- G. Guhados, W. Wan and J. L. Hutter, Langmuir, 2005, 21, 6642–6646 CrossRef CAS PubMed
.
- H. Backdahl, G. Helenius, A. Bodin, U. Nannmark, B. R. Johansson, B. Risberg and P. Gatenholm, Biomaterials, 2006, 27, 2141–2149 CrossRef PubMed
.
- A. Nakayama, A. Kakugo, J. P. Gong, Y. Osada, M. Takai, T. Erata and S. Kawano, Adv. Funct. Mater., 2004, 14(11), 1124–1128 CrossRef CAS
.
- R. Dash, M. Foston and A. J. Ragauskas, Carbohydr. Polym., 2013, 91, 638–645 CrossRef CAS PubMed
.
- T. Lu, Q. Li, W. Chen and H. Yu, Compos. Sci. Technol., 2014, 94, 132–138 CrossRef CAS
.
- Y. Cheng, J. Lu, S. Liu, P. Zhao, G. Lu and J. Chen, Carbohydr. Polym., 2014, 107, 57–64 CrossRef CAS PubMed
.
- C. Gao, T. Yan, K. Dai and Y. Wan, Cellulose, 2012, 19, 761–768 CrossRef CAS
.
- P. Tingaut, T. Zimmermann and G. Sebe, J. Mater. Chem., 2012, 22, 20105–20111 RSC
.
- S.-T. Chang, L.-C. Chen, S.-B. Lin and H.-H. Chen, Food Hydrocolloids, 2012, 12, 137–144 CrossRef
.
- Z. Dang, PhD thesis, Georgia Institute of Technology, 2007
.
- J. Röhrling, A. Potthast, T. Rosenau, T. Lange, G. Ebner, H. Sixta and P. A. Kosma, Biomacromolecules, 2002, 3(5), 959–968 CrossRef
.
- M. Azami, M. Rabiee and F. Moztarzadeh, Polym. Compos., 2010, 31(12), 2112–2120 CrossRef CAS
.
- M. Poletto, V. Pistor and A. J. Zattera, in Cellulose – Fundamental Aspects, ed. T. van de Ven and L. Godbout, InTech, Croatia, 2013, p. 200 Search PubMed
.
- M. Wada, J. Sugiyama and T. Okano, J. Appl. Polym. Sci., 1993, 49, 1491–1496 CrossRef CAS
.
- T. Zimmermann, E. Pohler and T. Geiger, Adv. Eng. Mater., 2004, 6(9), 754–761 CrossRef
.
- X. Zhang, Y. Yang, J. Yao, Z. Shao and X. Chen, ACS Sustainable Chem. Eng., 2014, 2, 1318–1324 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |