Noncovalent dispersion of multi-walled carbon nanotubes with poly(tert-butyl methacrylate) modified hyperbranched polyethylene for flexible conductive films†
Abstract
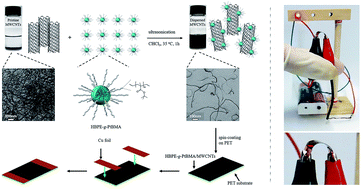
Carbon nanotubes (CNTs) have been extensively utilized in flexible electronics. However, further applications of CNTs are limited by their poor solubility in solvents. To overcome this obstacle, poly(tert-butyl methacrylate) modified hyperbranched polyethylene (HBPE-g-PtBMA) was employed. The HBPE core was designed to endow HBPE-g-PtBMA with a hyperbranched structure while the methyl groups of poly(tert-butyl methacrylate) provide CH–π interactions between HBPE-g-PtBMA and CNTs. Besides, the relative high melting endotherm of PtBMA segments enabled HBPE-g-PtBMA with feasibility to fabricate practical HBPE-g-PtBMA/MWCNTs conductive films. The bundled MWCNTs were found to be dispersed efficiently by HBPE-g-PtBMA into individual tubes with a maximum concentration of 455 mg L−1. Furthermore, fabrication of a conductive film by spin-coating the stable MWCNTs dispersion onto the PET substrate was explored. The conductive film was found to have good conductivity (13.14 S cm−1) and flexibility, which might have potential applications in flexible electronics.

 Please wait while we load your content...
Please wait while we load your content...