The access of Trichoderma reesei 6A to cellulose is blocked by isolated hemicelluloses and their derivatives in biomass hydrolysis†
Donglin Xin,
Ming Yang,
Xiang Chen and
Junhua Zhang*
College of Forestry, Northwest A&F University, Yangling 712100, Shaanxi, China. E-mail: junhuazhang@nwsuaf.edu.cn
First published on 28th July 2016
Abstract
During the conversion of lignocellulosic biomass to biochemical products, the end-products in the hydrolysate significantly inhibit cellulase efficiency and result in a low conversion yield. Our groups recently reported that cellobiohydrolase I (CBH I), the major enzyme in the cellulase mixture secreted by Trichoderma reesei, is susceptible to inhibition by xylan oligomers. In this work, effects of mannan with different structural properties, xylan and xylo-oligosaccharides (XOS) with different chain lengths on the hydrolytic action of CBHII, another key protein in commercial cellulase preparation, were investigated. Mannan and xylan oligomers blocked the adsorption of CBHII onto the cellulose surface and decreased the hydrolytic efficiency of CBHII. The ability of mannan in hindering the interaction of CBHII with cellulose varied with the physical properties of mannan. Mannan with high viscosity and galactose side units showed a stronger inhibitory effect on the efficiency of CBHII as compared with those with low viscosity and galactose side units. The inhibitory effect of XOS on the CBHII action decreased with the reduction of XOS chain length. Aside from CBHII, synergism between CBHI and CBHII was strongly inhibited by xylan and XOS. The results thus indicated the necessity of producing highly active CBHs that could minimize the product inhibition of cellulase in cellulose conversion to sugars.
Introduction
Utilization of renewable lignocellulosic biomass for production of biochemical products, such as various chemicals and biofuels, has drawn intensive research attention in the last two decades.1–3 The production of biofuels from lignocellulosic materials needs three main steps: pretreatment, enzymatic hydrolysis, and fermentation. Among the three steps, enzymatic hydrolysis is considered as one of the major limiting steps due to the recalcitrant and complex structure of the lignocelluloses.3,4 Efficient hydrolysis of cellulose requires the synergistic action of endoglucanases (EG), which randomly cleave internal bonds of the cellulose polymer, and exoglucanases or cellobiohydrolases (CBH), which act on the reducing and non-reducing ends, releasing cellobiose and cello-oligosaccharides. Finally, β-glucosidase (βG) hydrolyzes cellobiose to glucose. CBHI is the main protein in commercial cellulase preparations and regularly comprises about 60% of the total secreted protein by Trichoderma reesei, which is an industrially important cellulolytic filamentous fungus.5 CBHI catalyzes the release of cellobiose from the reducing chain ends in cellulose and is generally recognized as a key enzyme in the degradation of crystalline cellulose. CBHII acts on the non-reducing ends of cellulose chains, which is another key protein in commercial cellulase preparations and comprises about 15% of the total secreted protein by T. reesei.5 A synergistic action of CBHI and CBHII of T. reesei in cellulose hydrolysis has been observed6,7 and it is of great importance in releasing cellobiose from cellulose.In the enzymatic hydrolysis of lignocellulosic biomass to fermentable sugars, the major sugars in the hydrolysate are glucose, xylose, mannose, cello-oligosaccharides, xylo-oligosaccharides (XOS), manno-oligosaccharides, and isolated hemicelluloses. Somewhat interesting, all of them were found to be inhibitors of cellulases.8 As shown in Fig. 1, it was worth noting that the inhibition was mainly focused on CBHs action. The substrate inhibition of cellobiose and glucose on action of CBHI and CBHII has been pointed out by many researchers.9–11 Xylan, the major hemicelluloses in hardwoods and annual plants, is found to be closely associated with cellulose, blocks the access of cellulose, and consequently limits the hydrolysis of cellulose.12,13 It has been reported by many authors that xylanase supplementation clearly increased cellulose hydrolysis in xylan-containing lignocellulosic materials.14–17 Xylobiose and xylotriose are released as main hydrolysis products of XOS from xylan in the hydrolysis of biomass by cellulolytic and xylanolytic enzymes.18,19 Various xylans and XOS were found to have an inhibitory effect on the hydrolysis of different cellulosic materials by cellulases.20–23 It has been reported that the hydrolytic efficiency of the individual cellulases, EGII, CBHI, and CBHII on cellulose in Avicel, nanocellulose, and hydrothermally pretreated wheat straw, decreased in the presence of soluble or insoluble xylan.22 Aside from xylan, XOS and xylose inhibited actions of CBHI and CBHII on cellulose as well.23 It has been observed that XOS with high degree of polymerization of 8 or low DP of 4 decreased the activity of CBHI from T. reesei,20 and xylobiose and xylotriose were competitive inhibitors of CBHI from T. aurantiacus.23 Recently, the negative effects of mannan and manno-oligosaccharides on the hydrolytic action of cellulases on cellulose have been noticed and competitive inhibition of CBHI from T. aurantiacus by manno-oligosaccharides has also been confirmed.24,25 It seems that the inhibitory effects of hemicelluloses and their derivatives on the hydrolytic action of CBHI on cellulose have been widely investigated. The inhibitory effect of xylan and its derivatives on the hydrolysis of cellulose by CBHII has been just preliminarily confirmed,22,23 however, mechanism behind the inhibition was not fully understood, such as, effect of hemicelluloses and their derivatives on the adsorption of CBHII to cellulose is not clarified yet. Additionally, effect of mannan, the major hemicelluloses in softwood, on the hydrolytic action of CBHII has not been studied before. Detailed information about the inhibition of CBHII by hemicelluloses and their derivatives could help us further understanding the inhibitory effect of hemicelluloses and their derivatives on enzymatic hydrolysis of lignocellulosic materials by cellulases and finding efficient method way to relieve the inhibitory effect.
In this work, effects of mannan, xylan, XOS, and xylose on the adsorption of CBHII to cellulose were determined. Xylan, XOS, and xylose on the synergism between CBHI and CBHII, and XOS with different chain length on hydrolytic action of CBHII were investigated. In addition, hemicellulases, including endo-mannanase, endo-xylanase, and β-xylosidase, were added to reduce the inhibitory effect of hemicelluloses and their derivatives.
Experimental
Materials
Microcrystalline cellulose (Avicel PH-101), cellulose fiber (medium), beechwood xylan, and xylose were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Linear mannan (L-Man, Lot131001a), high viscosity carob galactomannan (GalM-H, mannose to galactose ratio: 3.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, viscosity 11.2 dL g−1, Lot60305b), low viscosity carob galactomannan (GalM-L, mannose to galactose ratio: 3.76
1, viscosity 11.2 dL g−1, Lot60305b), low viscosity carob galactomannan (GalM-L, mannose to galactose ratio: 3.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, viscosity 3 dL g−1, Lot10501b), xylobiose (X2), xylotriose (X3), and xylotetraose (X4) were purchased from Megazyme (Bray, Wicklow, Ireland). Corn stover was collected from a local farm in Yangling, China. The corn stover was milled and sieved through a 60 mesh screen scale. This milled material (≤0.3 mm) was pretreated by 21% (w/v) aqueous ammonia (CS-AA) with a solid to liquid ratio of 1
1, viscosity 3 dL g−1, Lot10501b), xylobiose (X2), xylotriose (X3), and xylotetraose (X4) were purchased from Megazyme (Bray, Wicklow, Ireland). Corn stover was collected from a local farm in Yangling, China. The corn stover was milled and sieved through a 60 mesh screen scale. This milled material (≤0.3 mm) was pretreated by 21% (w/v) aqueous ammonia (CS-AA) with a solid to liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 at 50 °C for 24 h and 1% (w/v) dilute acid (CS-DA) with a solid to liquid ratio of 1
10 at 50 °C for 24 h and 1% (w/v) dilute acid (CS-DA) with a solid to liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 at 121 °C for 1 h. The pretreated corn stover was washed to neutral with distilled water and then air dry for further use. The chemical compositions of the materials were determined by the National Renewable Energy Laboratory Analytical Procedure.26 The contents of cellulose and xylan in Avicel were 91.3% and 1.2%, and in cellulose fiber 90.1% and 3.0%. The contents of cellulose, xylan, and lignin in CS-AA were 54.1%, 17.4%, and 5.6%, and in CS-DA were 47.4%, 7.2%, and 31.6%, respectively.
10 at 121 °C for 1 h. The pretreated corn stover was washed to neutral with distilled water and then air dry for further use. The chemical compositions of the materials were determined by the National Renewable Energy Laboratory Analytical Procedure.26 The contents of cellulose and xylan in Avicel were 91.3% and 1.2%, and in cellulose fiber 90.1% and 3.0%. The contents of cellulose, xylan, and lignin in CS-AA were 54.1%, 17.4%, and 5.6%, and in CS-DA were 47.4%, 7.2%, and 31.6%, respectively.
Enzymes
The purified CBHII originating from T. reesei was kindly provided by Prof. Matti Siika-aho from VTT (Finland). The CBHI (Cel7A) from T. aurantiacus, the βG (Cel3A) from Acremonium thermophilum, and the endoxylanase preparation (XYL) originating from Thermoascus aurantiacus, were produced in a genetically modified T. reesei strain where the genes cbh1, cbh2, egl1 and egl2, encoding for Cel7A, CBHII (Cel6A), EGI (Cel7B) and EGII (Cel5A), respectively, had been deleted as described elsewhere.27–29 All enzyme preparations were adjusted to pH 6.0 and treated at 60 °C for 2 h to inactivate the background T. reesei enzymes. These thermostable enzyme preparations were kindly provided by RoalOy (Rajamäki, Finland). The β-xylosidase preparation (βX) from Selenomonas ruminantium was purchased from Megazyme (Bray, Wicklow, Ireland). The activity of βX was 8335 nkat mL−1 (40 °C, pH 5.3 on pNP-β-D-xylanopyranoside) based on information provided by the supplier. Endo-1,4-β-mannanase from Aspergillus niger was purchased from Megazyme (Bray, Wicklow, Ireland). The activity of mannanase preparation was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nkat mL−1 (40 °C, pH 4.0 on carob galactomannan) based on the supplier's data. Protein was quantified by the Lowry method,30 using bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA) as standard.
000 nkat mL−1 (40 °C, pH 4.0 on carob galactomannan) based on the supplier's data. Protein was quantified by the Lowry method,30 using bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA) as standard.
Preparation of phosphoric acid swollen cellulose
Phosphoric acid swollen cellulose (PASC) was generated from Avicel PH-101 cellulose according to the procedure of Walseth.31 Avicel (10 g) was moistened with 10 mL distilled water to form cellulose-suspended slurry. Ice cold 85% phosphoric acid (500 mL) was slowly added to the slurry with vigorous stirring and left with occasional stirring. After 2 h, two liters of distilled water were added with vigorous stirring, resulting in a white cloudy precipitate. The precipitated cellulose was centrifuged at 5000 g and 4 °C for 20 min. The pellet was suspended by ice-cold water, followed by centrifugation to remove the supernatant containing phosphoric acid for four times. Approximately 500 mL of 2% Na2CO3 was added to neutralize the residual phosphoric acid. After 12 h, the pellet was suspended by distilled water and centrifuged until pH 5–7. The crystalline behaviour of Avicel before and after swelling with phosphoric acid was evaluated by X-ray diffraction using a Rigaku D/max–3C generator (Rigaku Corporation, Japan). It was observed that the crystallinity index of Avicel clearly decreased from 75.5% to 31.2% after swelling with phosphoric acid (Fig. S1 in the ESI†). The results indicating that the crystalline zone was strongly broken by phosphoric acid and a large proportion of amorphous components were present in the PASC. The regenerated amorphous cellulose slurry was kept at 4 °C with 0.02% sodium azide for further use.Preparation of xylo-oligosaccharides
The preparation of xylo-oligosaccharides (XOS) was produced from 5% birchwood xylan (Sigma Chemical Co., St. Louis, MO, USA) by a partially purified endoxylanase preparation from Nonomuraea flexuosa, as described by Zhang and Viikari.23 The hydrolysis was performed in 50 mM sodium citrate buffer at 50 °C for 1 h. After the hydrolysis, the hydrolysate was boiled for 10 min to stop the enzymatic hydrolysis. After cooling, the hydrolysate was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min and the protein in the hydrolysate was removed with an ultrafiltration membrane with a molecular weight cut-off of 10 kDa (Amicon, Millipore). The sample was then analyzed by high-performance anion exchange chromatography coupled with pulsed amperometric detection and thin-layer chromatography using Silica Gel 60 plates (Merck), as shown in our previous results.32 The results indicated that the hydrolysate contained 32.2% X2, 46.3% X3, a low amount of xylose, and some unidentified oligosaccharides with higher degree of polymerization. The hydrolysate was used as XOS for following experiment.
000 g for 10 min and the protein in the hydrolysate was removed with an ultrafiltration membrane with a molecular weight cut-off of 10 kDa (Amicon, Millipore). The sample was then analyzed by high-performance anion exchange chromatography coupled with pulsed amperometric detection and thin-layer chromatography using Silica Gel 60 plates (Merck), as shown in our previous results.32 The results indicated that the hydrolysate contained 32.2% X2, 46.3% X3, a low amount of xylose, and some unidentified oligosaccharides with higher degree of polymerization. The hydrolysate was used as XOS for following experiment.
Enzymatic hydrolysis
The hydrolysis of Avicel, PASC, cellulose fiber, CS-AA, and CS-DA by different cellulase preparations was carried out in tubes with a working volume of 1 mL in 50 mM sodium citrate buffer (pH 5.0) containing 0.02% NaN3 at 50 °C. The hydrolysis was conducted in a shaking incubator with a shaking speed of 200 rpm. Cellulase preparations contained different individual cellulase component, including CBHII, CBHI, and βG. L-Man, GalM-H, GalM-L, xylan, XOS, and xylose were added into the reaction system at the beginning of the enzymatic hydrolysis. Samples were withdrawn and boiled for 10 min to stop the enzymatic hydrolysis. After cooling, the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min and the supernatants were analyzed for glucose or reducing sugars. Two replicate tests were carried out in all hydrolysis experiments and average values are presented.
000 g for 10 min and the supernatants were analyzed for glucose or reducing sugars. Two replicate tests were carried out in all hydrolysis experiments and average values are presented.
Adsorption experiment
The CBHII (8 mg protein per g DM) was incubated with Avicel (10 mg mL−1) with continuous stirring by a magnetic stirring apparatus at 4 °C for 1 h. The adsorption experiment was performed at 4 °C to avoid hydrolysis. It is known that adsorption is a physical process. When the temperature increases to 40–50 °C, the enzyme will hydrolyze cellulose and thus disturb the adsorption process. Mannan, xylan, XOS, and xylose, with different concentrations were added into the systems at the beginning of the adsorption experiment. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min, the residual amount of protein of the CBHII in the supernatant was measured using the BCA method33 with BSA as the protein standard. The amount of the CBHII bound to Avicel was estimated from the difference between the amounts of protein in the supernatants before and after incubation. The adsorption of mannan onto Avicel was investigated by the incubation of mannan (1, 2.5, 5 mg mL−1) with Avicel (10 mg mL−1) in 50 mM sodium citrate buffer (pH 5.0) at 4 °C with continuous stirring by a magnetic stirring apparatus for 1 h. After incubation, the samples were centrifuged at 10
000 g for 10 min, the residual amount of protein of the CBHII in the supernatant was measured using the BCA method33 with BSA as the protein standard. The amount of the CBHII bound to Avicel was estimated from the difference between the amounts of protein in the supernatants before and after incubation. The adsorption of mannan onto Avicel was investigated by the incubation of mannan (1, 2.5, 5 mg mL−1) with Avicel (10 mg mL−1) in 50 mM sodium citrate buffer (pH 5.0) at 4 °C with continuous stirring by a magnetic stirring apparatus for 1 h. After incubation, the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 min and the supernatants were analyzed for total polysaccharides by phenol-sulfuric acid method34 with mannose as standard. All adsorption experiments were done in duplicates and average values are presented.
000 g for 10 min and the supernatants were analyzed for total polysaccharides by phenol-sulfuric acid method34 with mannose as standard. All adsorption experiments were done in duplicates and average values are presented.
Carbohydrate analysis
Reducing sugars in XOS were analyzed by the dinitrosalicylic acid method with xylose as standard.35 The amounts of glucose and xylose in the enzymatic hydrolysate were determined using a HPLC system (Hitachi L-2000, Hitachi Corp., Japan). The system equipped with a refractive index detector (Hitachi Corp., Japan) and an autosampler (Hitachi Corp., Japan). Ion moderated partition chromatography column (Aminex column HPX-87H) with cation H micro-guard cartridge was used. The column was maintained at 45 °C with 5 mM H2SO4 as the eluent at a flow rate of 0.5 mL min−1. Before injection, samples were filtered through 0.22 μm MicroPES filters, and a volume of 20 μL was injected. Peaks were detected by refractive index and were identified and quantified by comparison to retention times of authentic standards (D-glucose and D-xylose).In this work, both Avicel and cellulose fiber were assumed as pure cellulose. Therefore, in the calculation of hydrolysis yield, Avicel, cellulose fiber, or PASC was considered as 100% cellulose, as shown in the following equation:
| Hyrolysis yield (%) = (amount of glucose released × 0.9)/(amount of Avicel, cellulose fiber or PASC) × 100 |
The glucose yield in the hydrolysis of corn stover was calculated as the following equation:
| Glucose yield (%) = (amount of glucose released × 0.9)/(theoretical amount of cellulose in substrates) × 100 |
The degree of inhibition was evaluated as the following equation.
| Degree of inhibition (%) = (Y0 − Yi)/Y0 × 100 |
Results and discussion
Effect of hemicelluloses and their derivatives on adsorption of CBHII on cellulose
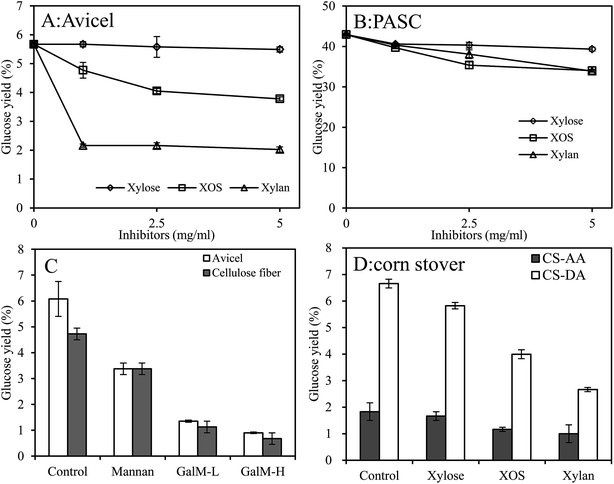
It is known that the accessibility of cellulose to cellulases significantly dominates the hydrolysis efficiency of cellulases.36 Therefore, the effect of the xylan and its derivatives on the interaction between cellulose and the CBHII was investigated by detecting the amount of the CBHII adsorbed on Avicel (Fig. 2A). In the absence of the inhibitors, above 80% of the CBHII protein was adsorbed on Avicel after 1 h incubation. However, after supplementation of 5 mg mL−1 xylan or XOS, the amount of the adsorbed protein decreased to different extents. The results indicated that the presence of xylan or XOS decreased the accessibility of cellulases to cellulose. It was also noticed that xylan showed stronger ability in weakening the adsorption of the CBHII to cellulose as compared with XOS, which was in good agreement with previous results that xylan exhibited stronger inhibitory effect on enzymatic hydrolysis of cellulose by CBHII than XOS.22 Our groups have been confirmed that xylan could adsorb on cellulose, following the Langmuir-type isotherm very well.37 The strong ability of xylan in coating cellulose surfaces inevitably decreased the access of CBHII to cellulose. For XOS, the possible reason for the phenomenon could be due to the bonding of XOS to active sites of the CBHII and thus reduced the binding site of the enzyme with cellulose. However, xylose showed negligible effect in decreasing the adsorption of CBHII to Avicel. The results were in good agreement with previous results in which low concentration xylose (10 mg mL−1) did not show a decrease in cellulase binding to substrate.38 However, high concentration of xylose (150 mg mL−1) showed strong ability in preventing cellulase adsorption to substrate.38 It was deduced that addition of monosaccharides affected the viscosity of solution and retarded the diffusion of cellulase to cellulose.38 Low concentration of xylose weakly increased the viscosity of hydrolysate and hence had negligible effect on the interaction of cellulase to substrate, which could the possible reason for the phenomenon. The results thus revealed that the inhibitory effect of xylan and its derivatives on CBHII hydrolysis was, at least, partially attributed to lowering the access of the CBHII to cellulose.However, aside from xylan, there is another hemicellulose that is widely present in the lignocellulosic materials. The hemicellulose, which is called mannan, is the major composition of hemicelluloses in softwood. The structure of mannan is so similar with xylan that analogous inhibitory effect of mannan on CBHII hydrolytic action may be existed. However, the possible phenomenon has not been fully investigated. In order to fill this gap, effect of L-Man, GalM-L, and GalM-H on the adsorption of CBHII on cellulose was investigated (Fig. 2B).
After addition of 5 mg mL−1 L-Man, GalM-L, and GalM-H, the adsorption of CBHII on cellulose decreased from 82.9% to 65.5%, 20.3%, and 13.4%, showing strong ability of mannan in reduction access of CBHII to cellulose. Similar to xylan, the negative effect of mannan on CBHII adsorption could be resulted from the coverage of mannan on cellulose and blocked the interaction of CBHII with cellulose. Therefore, the adsorption of mannan on Avicel was further investigated (Fig. 2C). It was observed that mannan could adsorb onto the surface of Avicel and the adsorption effect intensified with the increasing dosages of added mannan. When 5 mg mL−1 mannans were added in the systems, the amounts of L-Man, GalM-L, and GalM-H that adsorbed on the surface of Avicel were 15.6, 64.6, and 112.6 mg g−1 dry matter (DM), respectively. For L-Man, thus low adsorption ability onto cellulose could be mostly attributed to the fact that the mannan used in this work was a mixture of soluble and insoluble fractions. In this work, the amount of the mannan bound to Avicel was estimated from the difference between the amounts of mannan in the supernatants before and after incubation. However, the insoluble fractions of mannan could also precipitate on the surface of cellulose and block the adsorption of CBHII to cellulose. The results further indicated that the adsorption ability of mannan on cellulose varied with the structure and properties of mannan. Mannan with high viscosity and galactose side units showed stronger ability in precipitating on the surface of cellulose than those with low viscosity and galactose side units. It was worth noting the tendency was in good agreement with the results above, in which GalM-H with high viscosity and galactose side units exhibited stronger ability in reducing the access of CBHII to cellulose than GalM-L and L-Man. The results thus revealed that not only xylan, but also mannan could adsorb onto cellulose surfaces and blocked the interaction of CBHII with cellulose.
Effect of chain length of XOS on hydrolysis of cellulose by CBHII
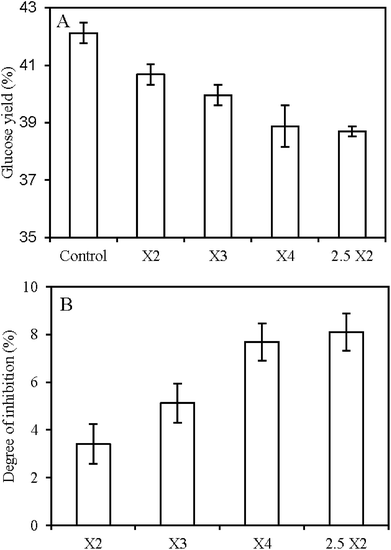
In above sections, the XOS used in the work of Fig. 2A was a mixture of xylobiose (X2), xylotriose (X3), and some unidentified oligosaccharides with higher degree of polymerization. However, in order to deeply explore the inhibition mechanism of XOS on CBHII, it is necessary to investigate the effect of pure substances of XOS on CBHII hydrolytic action. Due to the fact that the main XOS derived from xylan in the lignocellulosic biomass by xylanase were short chain oligosaccharides, hence, pure XOS, such as X2, X3, X4, were added into the reaction system during the hydrolysis of PASC by CBHII (Fig. 3). The βG preparation was supplemented to convert the cellobiose released by the CBHII to glucose to avoid the end product inhibition of cellobiose on CBHII. And the hydrolytic action of βG was not affected by xylan, XOS, and xylose.21 The addition of X2, X3, and X4 overall reduced the glucose yields of PASC by the CBHII, further revealing the inhibitory effect of XOS on the CBHII action. As expected, higher amount of X2 resulted in higher degree of inhibition in the enzymatic hydrolysis. In addition, the inhibitory effect of XOS increased with the increase of XOS chain length, as can be seen by comparison of their degrees of inhibition (Fig. 3B). In this work, X2–X4 were dosed at the same mass concentration (1 mg mL−1), which indicated that less molar concentration of X4 (1.8 mM) was dosed than that of X2 (3.5 mM). Stronger inhibitory effect of X4 was obtained with less molar concentration proposed that X4 might have stronger interaction with the CBHII than X2, which decreased the activity of the CBHII. Such interaction might be the binding of XOS to the active sites of the CBHII as competitive inhibitors. In previous results of Kumar and Wyman,14 they found that the increase of XOS concentration and chain length resulted in stronger inhibitory effect on initial hydrolysis rate of Avicel by commercial cellulases. The phenomenon could be partly explained by stronger inhibition of XOS with higher length on CBHII hydrolytic action, as shown in the results here.Effect of xylan, XOS, and xylose on the hydrolysis of cellulose by CBHI and CBHII
Inhibitory effects of isolated xylan, XOS, and xylose on hydrolytic action of individual CBHI and CBHII on various celluloses had been confirmed by previous results.22,23 However, in a real biomass enzymatic hydrolysis system, exo–exo synergism between CBHI and CBHII plays a key role in catalyzing the release of cellobiose from the reducing and non-reducing chain ends in cellulose and is a major step in the hydrolysis of cellulose by commercial cellulase preparations.6,7 The effects of xylan, XOS, and xylose on the synergism between CBHI and CBHII on cellulose are still unclear. Therefore, xylan, XOS, and xylose were added in the hydrolysis of Avicel and PASC by the CBHI and CBHII to investigate the inhibitory effects of the three inhibitors (Fig. 4). After addition of xylan, XOS, and xylose (5 mg mL−1), the glucose yields of Avicel decreased from 19.8% to 2.5%, 9.7%, and 19.1%, respectively. Such severe inhibitory effects of xylan were also noticed in the hydrolysis of PASC (Fig. 4B). It was observed that xylan, XOS, and xylose resulted in degrees of inhibition of 87.3%, 50.9%, and 3.6% in the hydrolysis of Avicel by CBHI and CBHII. However, in the hydrolysis of Avicel by individual CBHI and CBHII, the addition of equal amount of xylan decreased the glucose yields by 42.7% and 63.9% (Table 1), which was much lower than those in hydrolysis of Avicel by mixture of CBHI and CBHII. A similar phenomenon was observed when XOS and xylose were added in the hydrolysis system. The results thus revealed that xylan and its derivatives exhibited stronger inhibitory effect on the synergism between CBHI and CBHII than on individual CBHI or CBHII. In the hydrolysis of lignocelluloses by commercial cellulases, both CBHI and CBHII are included and severe inhibitory effect of xylan and XOS might be existed. Thus, completely hydrolysis of xylan and XOS into xylose was a necessity to alleviate the inhibitory effects.| Enzyme | Degree of inhibition (%) | ||
|---|---|---|---|
| Xylan | XOS | Xylose | |
| CBHI | 42.7 ± 0.7 | 23.4 ± 1.3 | 2.3 ± 0.2 |
| CBHII | 63.9 ± 2.4 | 33.3 ± 2.1 | 3.1 ± 0.3 |
| CBHI + CBHII | 87.3 ± 1.8 | 50.9 ± 3.7 | 3.6 ± 0.1 |
Effect of xylan, XOS, and xylose on the hydrolysis of Avicel, PASC, and corn stover by CBHII
According to a rough estimation, the actual concentrations of hemicelluloses (xylan and mannan) or their derivatives may be in the range of 5–10 mg mL−1 when lignocellulosic materials is hydrolyzed at high solid concentrations, such as 10%. However, in our previous results, the inhibition of xylan, XOS, and xylose on hydrolytic action of CBHII was performed at a single low concentration (2 mg mL−1).13,14 In order to reflect the real reaction conditions, it is necessary to further investigate the effect of high concentrations of hemicelluloses and their derivatives on the hydrolysis of cellulose by CBHII. Therefore, isolated xylan, XOS, and xylose with different concentrations (1–5 mg mL−1), and mannan with different structure properties, were added in the hydrolysis of Avicel, PASC, CS-AA, CS-DA by the CBHII (Fig. 5). It was observed that the inhibitory effects on cellulose hydrolysis by CBHII increased with the concentrations of the added inhibitors. Glucose yields of Avicel after 24 h were as low as 2.0%, 3.8%, and 5.5% after addition of 5 mg mL−1 xylan, XOS, and xylose, respectively, which were lower than that of the control (5.7%) (Fig. 5A). In the hydrolysis of PASC, the addition of 5 mg mL−1 xylan, XOS, and xylose decreased the glucose yields from 42.9% to 33.9%, 34.0%, and 39.3%, respectively (Fig. 5B). It was observed that the addition of L-Man, GalM-L, and GalM-H clearly decreased the hydrolysis yields of cellulose in cellulose fiber from 9.5% to 6.8%, 2.3%, and 1.4% (Fig. 5C). A similar phenomenon was observed when Avicel was used as substrate. The results thus indicated the negative effect of mannan on the hydrolysis of cellulose by CBHII. Additionally, it was noticed that mannan with higher viscosity and galactose side units showed stronger inhibitory effect on the hydrolysis of cellulose by CBHII as compared with those with low viscosity and galactose side units. The major factor contributing to the inhibition of hemicelluloses and theirs derivatives on the hydrolysis of cellulose by CBHII could be the bounding effect of the inhibitors on the access of CBHII to cellulose surface, and consequently lowered the hydrolysis yields, as shown in Fig. 2.The degrees of inhibition by xylan, XOS, and xylose in the hydrolysis of Avicel and PASC were calculated to compare the inhibitory effect of the inhibitors on the CBHII (Table 2). In the hydrolysis of PASC, the degrees of inhibition by 5 mg mL−1 xylan, XOS, and xylose were 20.1%, 20.7%, and 8.4%, respectively. However, greater degree of inhibition by equal amounts of xylan and XOS were obtained in Avicel hydrolysis. After pretreatment by phosphoric acid, PASC had higher contents of amorphous cellulose and surface areas as compared to Avicel,39–41 which may leave a larger portion of cellulose uncovered by the added xylan, and leave high contents of amorphous cellulose and larger surface area accessible to the CBHII, thus resulting in a weaker inhibitory effect in the hydrolysis of PASC. It was also observed that both xylan and XOS exhibited relatively stronger degree of inhibition than xylose on the enzymatic hydrolysis. The inhibitory pattern was in good agreement with the previous results that xylan and XOS showed stronger inhibitory effect on the adsorption of CBHII to cellulose (Fig. 2).
| Substrate | Inhibitor | Degree of inhibition (%) | ||
|---|---|---|---|---|
| 1 mg mL−1 | 2.5 mg mL−1 | 5 mg mL−1 | ||
| Avicel | Xylan | 61.51 ± 3.7 | 61.51 ± 3.7 | 63.93 ± 3.4 |
| XOS | 15.91 ± 1.3 | 28.62 ± 1.1 | 33.31 ± 1.1 | |
| Xylose | 1.57 ± 0.0 | 2.31 ± 0.2 | 3.11 ± 0.0 | |
| PASC | Xylan | 6.33 ± 0.4 | 11.81 ± 0.5 | 20.11 ± 0.9 |
| XOS | 8.21 ± 0.6 | 17.62 ± 0.5 | 20.72 ± 0.5 | |
| Xylose | 5.42 ± 0.6 | 6.13 ± 0.6 | 8.42 ± 0.6 | |
The inhibitory effects of xylan, XOS, and xylose in the hydrolysis of CS-AA and CS-DA were investigated (Fig. 5D).
The hydrolysis yields of cellulose in the CS-AA and CS-DA by the CBHII and βG were 1.8% and 6.6%, respectively. The low hydrolysis yield was mainly attributed to the absence of other cellulases and accessory enzymes, such as CBHI, EG and the steric hindrance of lignin and xylan. After the addition of xylan and XOS, the glucose yields of cellulose in CS-AA decreased from 1.8% to 1.0% and 1.2%, respectively. A similar phenomenon was observed when CS-DA was used as the substrate. The addition of xylan decreased the hydrolysis yields of cellulose in CS-AA and CS-DA by 44.4% and 59.7%, indicating the stronger inhibitory effect of xylan on the hydrolysis of CS-DA by CBHII. As mentioned in material section, the content of cellulose, xylan, and lignin in CS-DA were 47.4%, 7.2%, and 31.6%, and in CS-DA 54.1%, 17.4%, and 5.6%, respectively. Thus different contents of xylan and lignin in the two materials could be the possible reason for the diverse inhibitory degree of xylan on the hydrolysis of CS-AA and CS-DA by CBHII. Further studies should be performed to deeply investigate which fractions affect the inhibitory effect most, and the mechanism behind the phenomenon. It was observed that xylose showed weaker inhibitory effect in the hydrolysis of different cellulosic materials by the CBHII as compared with xylan or XOS. Thus, the xylan in substrates or XOS produced in the enzymatic hydrolysis should be converted into xylose to reduce the inhibitory effects on the CBHII.
Reduction of hemicelluloses inhibition by hemicellulolytic enzymes
Hemicellulolytic enzymes, endomannanase, endoxylanase (XYL), and βX, were added to hydrolyze hemicelluloses to try to overcome their inhibitory effects in the hydrolysis of different lignocellulosic materials by CBHII (Table 4 and Fig. 6). In the hydrolysis of PASC by the CBHII, the glucose yield decreased from 38.3% to 31.6% after the addition of xylan (Fig. 6A). After supplementation of the XYL with xylan, the glucose yield clearly increased to 46.2%. However, the glucose yield was further increased to 47.8% by supplementing of the XYL and βX with xylan. A similar phenomenon was observed in the hydrolysis of Avicel by the CBHI and CBHII. The results revealed that the XYL played a more important role in the reduction of inhibitory effect of xylan on the enzymatic hydrolysis than the βX. The possible reasons for the positive effect of the XYL could be attributed to the removal of adsorbed xylan on the surfaces of cellulose, the solubilization of xylan present in the substrate, and consequently increased the access of the CBH to cellulose. Additionally, about half of the xylan was converted to less inhibitory xylose by the XYL (Table 3), which could be another possible mechanism for the positive effect of xylanolytic enzymes. The addition of XOS expectedly decreased the glucose yield of PASC by CBHII and Avicel by CBHI and CBHII from 38.3% and 32.0% to 31.6% and 15.1%, respectively. However, after supplementation of βX, the glucose yields increased to 33.2% and 22.3%. It was observed that half of the XOS was converted to xylose by the βX (Table 3). The results thus indicated that xylan and XOS inhibition to enzymatic hydrolysis of cellulose by CBHII could be alleviated by converting them into less inhibitory xylose. As shown in Table 4, after addition of mannanase with L-Man, GalM-L, and GalM-H, the hydrolysis yields of cellulose in Avicel were 9.4%, 7.9%, and 8.1%, which were close to the control (10.6%). The results indicated that supplementation with mannanase significantly reduced the inhibitory effect of mannan on cellulose hydrolysis. The mannan used in this work is mainly composed of mannose and galactose. However, in real softwood, aside from mannose and galactose, glucose unit is present in the backbone polymer chain of galactoglucomannan. Therefore, the compositions and structure of mannan in this work is different with that in native softwood, which may result in different inhibitory effects on the hydrolysis of cellulose by cellulases. In order to fully understand the inhibitory effect of mannan on the hydrolysis of cellulose in softwood, further studies using galactoglucomannan extracted from softwood should be performed in further studies.| PASC | Avicel | CS-AA | CS-DA | |
|---|---|---|---|---|
| a bdl: below detection limit. | ||||
| Xylan | bdl | bdl | bdl | bdl |
| Xylan + XYL | 2.31 ± 0.2 | 1.82 ± 0.5 | 2.52 ± 0.1 | 2.42 ± 0.2 |
| Xylan + XYL + βX | 3.61 ± 0.0 | 3.81 ± 0.0 | 4.11 ± 0.2 | 3.92 ± 0.1 |
| XOS | 0.22 ± 0.0 | 0.24 ± 0.0 | 0.41 ± 0.0 | 0.31 ± 0.0 |
| XOS + βX | 2.62 ± 0.1 | 2.43 ± 0.1 | 2.82 ± 0.1 | 2.51 ± 0.0 |
| XYL | — | — | 0.23 ± 0.0 | 0.04 ± 0.0 |
| XYL + βX | — | — | 0.44 ± 0.0 | 0.06 ± 0.0 |
| Enzyme | Control | L-Man | GalM-L | GalM-H |
|---|---|---|---|---|
| CBHII | 10.58 ± 0.7 | 5.85 ± 0.3 | 2.48 ± 0.2 | 2.03 ± 0.2 |
| CBHII + mannanase | 9.98 ± 0.4 | 9.45 ± 0.9 | 7.88 ± 0.7 | 8.12 ± 0.9 |
Effect of xylanolytic enzymes on the reduction of xylan and XOS inhibition in the hydrolysis of real substrates, CS-AA and CS-DA, by the CBHII was also investigated (Fig. 6B). Addition of the XYL largely increased the glucose yields of CS-AA and CS-DA from 2.7% and 5.0% to about 9.6% and 11.0%, respectively, due to the solubilization of xylan in the substrates and the increase of the accessibility of the CBHII to cellulose in the substrates. As expected, the addition of xylan decreased the glucose yields in the hydrolysis of the two substrates by the CBHII. After supplementation of xylanolytic enzymes with xylan, the glucose yields of CS-AA and CS-DA were increased to about 10% due to the solubilization of xylan in substrates and xylan on the surface, approximately equaling to the hydrolysis system with xylan addition. The results indicated that the addition of the xylanolytic enzymes, such as the XYL and βX, could overcome the inhibitory effect of the xylan in the hydrolysis of real substrates. In addition, the alleviation of XOS inhibition to the enzymatic hydrolysis by βX was also noticed in the hydrolysis of the pretreated corn stover substrates, which was due to the conversion of XOS into xylose (Table 3).
Previously, supplementation of xylanases and β-xylosidases clearly increased the hydrolysis yields of cellulose in different corn stover substrates, and the removal or conversion of xylan and XOS to xylose was suggested to be the possible reason for the positive effect.42 The results in this work confirmed that the XYL and βX could solubilize xylan, convert XOS to xylose, and finally overcome the inhibitory effects of xylan and XOS on the hydrolysis of cellulose by key cellulase of the CBHII. In addition, the conversion of xylan and XOS to xylose could also alleviate inhibitory effects of xylan and XOS on CBHI.43 Enzymatic hydrolysis with a high solid loading is a direct and convenient method to obtain a high concentration of sugars and can potentially reduce production costs of biofuels and chemicals. Thus, the inhibition of xylan and XOS on CBH hydrolysis would be more severe due to the high solid loading, and complete conversion of xylans in substrates into end product of xylose would be of great importance. Complete hydrolysis of xylans involve the synergistic action of main chain cleaving enzymes, including endoxylanase and xylosidase, and side group cleaving enzymes, including α-L-arabinofuranosidases, α-glucuronidases, acetyl xylan esterases, and feruloyl esterases. However, efficient xylanolytic enzyme cocktail is dependent on the xylan structure in lignocelluloses and the method of pretreatment, which can alter the structure of xylan in substrates.
Conclusions
The adsorption of mannan and xylan onto the surfaces of cellulose retarded the access of CBHII to cellulose, which provided helpful information about the negative effects of isolated hemicelluloses on cellulose hydrolysis. Additionally, it was observed that the negative effect on CBHII varied with the structure properties of mannan and xylan oligomers. A stronger inhibitory effect by xylan and xylan oligomers was observed on the synergism between CBHI and CBHII than on individual CBHI or CBHII. The results reported herein expand knowledge about sugars inhibition on cellulases during the enzymatic hydrolysis process. The results reported here greatly suggested that highly active CBHs and hemicellulolytic enzymes, such as mannanase, and xylanase should be produced in further enzyme cocktails to relieve the product inhibition and realize the highly efficient production of fermentable sugars with low costs.Acknowledgements
This work was supported by the Natural Science Foundation of China (number: 31270622). The authors are grateful to Prof. Liisa Viikari (University of Helsinki, Finland) and RoalOy (Rajamäki, Finland) for providing the CBHI, CBHII, βG, and XYL. Matti Siika-aho (VTT, Finland) is thanked for supplying the CBHII.References
- Y. Wang, S. De and N. Yan, Chem. Commun., 2016, 52, 6210–6224 RSC.
- X. Chen and N. Yan, Catal. Surv. Asia, 2014, 18, 164–176 CrossRef CAS.
- M. E. Himmel, S. Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804–807 CrossRef CAS PubMed.
- A. Barakat, C. Mayer-Laigle, A. Solhy, R. A. Arancon, H. De Vries and R. Luque, RSC Adv., 2014, 4, 48109–48127 RSC.
- K. McFarland, D. Hanshu, S. Teter, E. Vlasenko, X. Feng and J. Cherry, Industrial Application of Enzymes on Carbohydrate-Based Material, 2007, vol. 972, pp. 19–31 Search PubMed.
- B. Henrissat, H. Driguez, C. Viet and M. Schülein, Nat. Biotechnol., 1985, 3, 722–726 CrossRef CAS.
- J. Medve, J. Ståhlberg and F. Tjerneld, Biotechnol. Bioeng., 1994, 44, 1064–1073 CrossRef CAS PubMed.
- L. Sun and J. Zhang, Biomass Chem. Eng., 2012, 46, 39–44 CrossRef (in chinese).
- R. M. Bezerra and A. A. Dias, Appl. Biochem. Biotechnol., 2005, 126, 49–59 CrossRef CAS PubMed.
- X. Huang and M. H. Penner, J. Agric. Food Chem., 1991, 39, 2096–2100 CrossRef CAS.
- P. Väljamäe, V. Sild, G. Pettersson and G. Johansson, Eur. J. Biochem., 1998, 253, 469–475 Search PubMed.
- S.-Y. Ding and M. E. Himmel, J. Agric. Food Chem., 2006, 54, 597–606 CrossRef CAS PubMed.
- H. H. Yoon, Korean J. Chem. Eng., 1998, 15, 631–636 CrossRef CAS.
- R. Kumar and C. E. Wyman, Biotechnol. Bioeng., 2009, 102, 457–467 CrossRef CAS PubMed.
- K. Ohgren, R. Bura, J. Saddler and G. Zacchi, Bioresour. Technol., 2007, 98, 2503–2510 CrossRef PubMed.
- M. J. Selig, E. P. Knoshaug, W. S. Adney, M. E. Himmel and S. R. Decker, Bioresour. Technol., 2008, 99, 4997–5005 CrossRef CAS PubMed.
- R. Agrawal, R. Gaur, A. Mathur, R. Kumar, R. P. Gupta, D. K. Tuli and A. Satlewal, RSC Adv., 2015, 5, 71462–71471 RSC.
- J. Zhang, P. Tuomainen, M. Siika-Aho and L. Viikari, Bioresour. Technol., 2011, 102, 9090–9095 CrossRef CAS PubMed.
- C. Zilliox and P. Debeire, Enzyme Microb. Technol., 1998, 22, 58–63 CrossRef CAS.
- M. J. Baumann, K. Borch and P. Westh, Biotechnol. Biofuels, 2011, 4, 45 CrossRef CAS PubMed.
- Q. Qing, B. Yang and C. E. Wyman, Bioresour. Technol., 2010, 101, 9624–9630 CrossRef CAS PubMed.
- J. Zhang, M. Tang and L. Viikari, Bioresour. Technol., 2012, 121, 8–12 CrossRef CAS PubMed.
- J. Zhang and L. Viikari, Bioresour. Technol., 2012, 117, 286–291 CrossRef CAS PubMed.
- R. Kumar and C. E. Wyman, Biotechnol. Bioeng., 2014, 111, 1341–1353 CrossRef CAS PubMed.
- D. Xin, X. Ge, Z. Sun, L. Viikari and J. Zhang, Enzyme Microb. Technol., 2015, 68, 62–68 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Report N. TP-510-42618, 2011, p. 17.
- S. Leskinen, A. Mantyla, R. Fagerstrom, J. Vehmaanpera, R. Lantto, M. Paloheimo and P. Suominen, Appl. Microbiol. Biotechnol., 2005, 67, 495–505 CrossRef CAS PubMed.
- P. L. Suominen, A. L. Mäntylä, T. Karhunen, S. Hakola and H. Nevalainen, Mol. Genet. Genomics, 1993, 241, 523–530 CrossRef CAS PubMed.
- J. Vehmaanperä, M. Alapuranen, T. Puranen, M. Siika-aho, J. Kallio, S. Hooman, S. Voutilainen, T. Halonen and L. Viikari, Patent, WO.2007071818, 2007.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- C. S. Walseth, Tappi, 1952, 35, 228–233 CAS.
- J. Zhang, M. Siika-aho, T. Puranen, M. Tang, M. Tenkanen and L. Viikari, Biotechnol. Biofuels, 2011, 4, 1 CrossRef PubMed.
- P. K. Smith, R. I. Krohn, G. Hermanson, A. Mallia, F. Gartner, M. Provenzano, E. Fujimoto, N. Goeke, B. Olson and D. Klenk, Anal. Biochem., 1985, 150, 76–85 CrossRef CAS PubMed.
- M. Dubois, K. Gilles, J. Hamilton, P. Rebers and F. Smith, Nature, 1951, 168, 167 CrossRef CAS PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- T. Jeoh, C. I. Ishizawa, M. F. Davis, M. E. Himmel, W. S. Adney and D. K. Johnson, Biotechnol. Bioeng., 2007, 98, 112–122 CrossRef CAS PubMed.
- X. Wang, K. Li, M. Yang and J. Zhang, Carbohydr. Polym., 2016, 148, 362–370 CrossRef CAS PubMed.
- C.-w. C. Hsieh, D. Cannella, H. Jørgensen, C. Felby and L. G. Thygesen, J. Agric. Food Chem., 2014, 62, 3800–3805 CrossRef CAS PubMed.
- G. Moxley, A. R. Gaspar, D. Higgins and H. Xu, J. Ind. Microbiol. Biotechnol., 2012, 39, 1289–1299 CrossRef CAS PubMed.
- J. Stone, A. Scallan, E. Donefer and E. Ahlgren, Adv. Chem. Ser., 1969, 95, 219–241 CrossRef CAS.
- Y. H. Zhang, J. Cui, L. R. Lynd and L. R. Kuang, Biomacromolecules, 2006, 7, 644–648 CrossRef CAS PubMed.
- Q. Qing and C. E. Wyman, Biotechnol. Biofuels, 2011, 4, 18 CrossRef CAS PubMed.
- D. Xin, Z. Sun, L. Viikari and J. Zhang, Ind. Crops Prod., 2015, 74, 209–217 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD analysis of Avicel and phosphoric acid swollen cellulose (PASC). See DOI: 10.1039/c6ra14617a |
| This journal is © The Royal Society of Chemistry 2016 |