The role of the KRSIK motif of human angiogenin in heparin and DNA binding†
Abstract
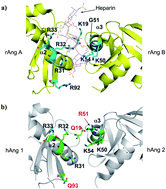
The positively charged surface with a 50KRSIK54 motif is the main interaction site of hAng for both heparin and DNA binding, providing an insight into the potential role of the 50KRSIK54 motif for the internalization and promoter binding of hAng, which is essential for the regulation of angiogenesis.

 Please wait while we load your content...
Please wait while we load your content...