A novel insensitive cocrystal explosive BTO/ATZ: preparation and performance†
Abstract
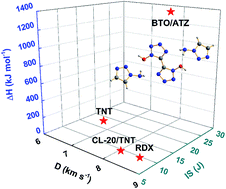
In order to explore new applications of novel nitrogen-rich energetic materials based on 1H,1′H-5,5′-bitetrazole-1,1′-diolate (BTO), aside from BTO based energetic-salts, a cocrystalline energetic material composed of BTO and 1-amino-1,2,3-triazole (ATZ) in a 1 : 2 molar ratio was synthesized, which is the first reported BTO based material with a cocrystal structure. The structure of the cocrystal was characterized using powder X-ray diffraction and single crystal X-ray diffraction, which indicate that the cocrystal is formed by intermolecular hydrogen bonding interactions, and is crystallized in the monoclinic system, space group C2/c, with a density of 1.697 g cm−3. The properties of the cocrystal including the thermal decomposition, sensitivity, and detonation performances are discussed in detail. Differential scanning calorimetry (DSC) and thermogravimetry/derivative thermogravimetry (TG/DTG) technologies were employed to determine the thermal decomposition behavior of the cocrystal, and the results are significantly different from the decomposition behavior of the co-formers. The enthalpy of formation was calculated as 1376.5 kJ mol−1, which is obviously higher than that of RDX. Sensitivity studies showed that the cocrystal has an impact sensitivity of 24 J, and so is insensitive to impact stimulation. In addition, the detonation pressure (P) and detonation velocities (D) of the cocrystal were predicted by using K–J equations, and the results obtained are 8088 m s−1 for detonation velocity and 28.1 GPa for detonation pressure, which are at the same level as RDX. Combining these advantages, this cocrystal possesses a promising future for use as a type of insensitive explosive. The discovery of the cocrystal contributes significantly to the expansion and application of the chemistry of 1H,1′H-5,5′-bitetrazole-1,1′-diolate.

 Please wait while we load your content...
Please wait while we load your content...