A facile synthesis method and electrochemical studies of a hierarchical structured MoS2/C-nanocomposite†
Zhenyou Liab,
Alexander Ottmanna,
Elisa Thauera,
Christoph Neefa,
Huazheng Saib,
Qing Suna,
Krzysztof Cendrowskie,
Hans-Peter Meyerd,
Yana Vaynzofac,
Ewa Mijowskae,
Junhui Xiang*b and
Rüdiger Klingelerac
aKirchhoff Institute of Physics, INF 227, Heidelberg University, Germany. E-mail: zhenyou.li@kip.uni-heidelberg.de; klingeler@kip.uni-heidelberg.de
bCollege of Materials Science and Opto-Electronic Technology, University of Chinese, Academy of Sciences, Yuquan Road 19A, Beijing 100049, China. E-mail: xiangjh@ucas.ac.cn
cCentre for Advanced Materials (CAM), INF 225, Heidelberg University, Germany
dInstitute of Earth Sciences, Heidelberg University, D-69120 Heidelberg, Germany
eDepartment of Nanotechnology, Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology, Piastow 45 Av., 70-311, Szczecin, Poland
First published on 29th July 2016
Abstract
A uniformly coated MoS2/carbon-nanocomposite with a three-dimensional hierarchical architecture based on carbonized bacterial cellulose (CBC) nanofibers is synthesized by a facile one-step hydrothermal method followed by thermal annealing at 700 °C in an Ar atmosphere. Strong hydrogen bonds between the Mo precursor and the BC nanofibers are found to be crucial for the in situ growth of MoS2 nanosheets on the nanofibers during the hydrothermal process. The fibrous structure was maintained and the connection between MoS2 and the nanofibers were strengthened in the sintering process, leading to an improved stability of the resulting nanocomposite upon electrochemical cycling. The low-cost and environmentally friendly 3D web-like structure enables binder-free and carbon-free electrodes for lithium-ion batteries, which exhibit high specific discharge capacities up to 1140 mA h g−1 at the C-rate of 1C without significant capacity fading for over 50 cycles. The porous conductive hierarchical structure of the composite endows excellent rate performance by avoiding the aggregation of the MoS2 nanosheets and by accommodating mechanical stress which appears upon electrochemical cycling.
Introduction
Molybdenum disulphide (MoS2) has attracted significant attention because of its unique properties and wide field of applications, which includes excellent electrochemical performance.1–6 Analogously to graphene, it has a two-dimensional layered structure, giving rise to unique electronic properties5,7,8 and it allows for the intercalation of lithium ions without obvious volumetric change.9–11 In LIBs, MoS2 can also react with Li to form metal Mo and Li2S during a conversion process, leading to a significant improvement in capacity (670 mA h g−1) as compared to intercalation based anode materials.12 This is not restricted to LIBs as MoS2 can also host Na ions which renders it a potential anode material for reversible sodium ion batteries as well.13 However, the electrochemical performance of bulk MoS2-based anodes still features disadvantages concerning practical application in LIBs. The large volume expansion accompanying the conversion reaction causes electrode pulverization and derogates the integrity of the active material and current collector.14 Poor cycling stability and rate performance is further increased by intrinsically poor electrical and ionic conductivities of the materials, which are further deteriorated by the structure destruction during the lithiation–delithiation process.9There are mainly two common approaches that have been successfully applied to overcome these issues in a variety of LIB electrode materials: downscaling of primary particles and fabrication of hierarchically structured hybrid materials.15 In the case of MoS2, various nanostructures (such as nanotubes, hollow spheres, core–shell structures etc.)9,12,16 have been produced aiming at accommodating mechanical strain upon electrochemical cycling as well as at shortening the transport distance of electrons and lithium ions. In addition, several approaches for synthesizing hierarchically structured MoS2/conductive material composites with nanomaterials like graphene, carbon nanotubes (CNTs) etc. have been reported.14,17–24 Chen et al.14 reported a L-cysteine-assisted method to synthesize MoS2/graphene composites with excellent cycling stability and high-rate capability. Also, cylindrical, nanostructured MoS2/CNT composites showed superior electrochemical performance in LIBs.17
In hierarchical composites, the individual 2D MoS2 nanosheets are to be preserved by the primary architectures, providing highly exposed surface area and large specific surface area for electrochemical reactions. The particular hierarchy and morphology is crucial for the electrochemical performance of the material. This also holds for preparation of the working electrode, which usually includes mixing with additional carbon and an organic binder. Even upon strong stirring it is difficult to achieve homogeneous anode slurries. Note, that strong stirring may destroy the nanostructure. Moreover, most of the applied binders are low conductivity polymers, which reduce the performance of the electrode. In this regards, it is most appealing to aim at electrodes free of binders and additional carbon. However, only very few studies on binder-free MoS2 anodes including nanostructured carbon have been reported so far.10,25,26
Herein, a facile synthetic method to fabricate a composite of carbonized bacterial cellulose (BC) nanofibers uniformly coated with MoS2 nanosheets (S-MoS2/BC) is presented. The strong interaction between the MoS2 nanosheets and the fibers is attributed to hydrogen bonds between the subsystems of the composite. This synthesis approach hence results in a stable and conductive 3D network of nanofibers, which fastens the electron and ion transport, prevents agglomeration of the nanosheets, and provides a large specific surface area. The novel architecture exhibits greatly improved cycling performance and rate performance with regard to the pure MoS2 assembly. The synthesis procedure mainly applies hydrothermal synthesis using the MoS2 precursors together with the BC matrix, which is a facile and easily scalable method. This method enables preparing binder-free electrodes without additional carbon, i.e. the pristine product material can be used in LIBs without further C-functionalisation. The straightforward synthesis approach applied here hence renders the material much more cost-efficient as compared to graphene and carbon nanotubes-based nanocomposites which require complex and expensive synthetic procedures.27
Experimental
Material synthesis
The bacterial cellulose (BC) nanofiber matrix was prepared from the delicious food Nata-de-coco (Kuangquan Food Co. Ltd).28 The Nata-de-coco were immersed in deionized water for 4 h to remove the sugar inside. The washed BC hydrogels were heated to 90 °C in a concentrated NaOH solution and were kept there for 6 h. Then, the hydrogels were washed for 10 h in a beaker with 100 ml water while stirring. The water was exchanged every 2 h. Afterwards, the MoS2/BC composites were produced by hydrothermal synthesis. 200 mg ammonium molybdate tetrahydrate (AMT, Sigma-Aldrich, 81.0–83.0% MoO3 basis) was dissolved in 50 ml deionized water. 200 mg thiourea (Sigma-Aldrich, 99%) was added subsequently. After that, the BC matrix was immersed into the solution under mild stirring overnight. Then, the solution as well as the BC matrix were transferred into a 50 ml stainless steel autoclave and heated in an electric oven at 200 °C for 24 h. In order to remove any remaining precursors and soluble impurities, the products were washed with deionized water for at least 12 h under magnetic stirring. The MoS2/BC composites were dried through a freeze-drying method for 24 h. The final product, the S-MoS2/BC composite, was prepared by sintering the MoS2/BC in a tube furnace at 700 °C for 10 h in Ar atmosphere. Pure MoS2 nanosheets which were studied as a reference were prepared by the same hydrothermal method without the BC matrix, followed by sintering.Characterization
X-Ray powder diffraction (XRD) was performed in Bragg–Brentano geometry (Bruker-AXS D8 ADVANCE ECO) applying Cu-Kα1 radiation (λ = 1.54056 Å). The step size Δ2θ was 0.02°. The morphology of the powder was characterized by means of a ZEISS Leo 1530 scanning electron microscope (SEM) after preliminary plasma sputtering of a 10 nm gold thin film on the samples in Ar atmosphere. The morphology of the samples was also investigated by a FEI Tecnai F30 transmission electron microscope (TEM) with a field emission gun operating at 200 kV and elemental distribution was studied by energy-dispersive X-ray spectroscopy (EDX). X-ray photoemission spectroscopy (XPS) was carried out in an ESCALAB 250Xi ultra-high vacuum system. Using an Al Kα radiation source (hν = 1486.6 eV), a 900 μm spot size and 20 eV pass energy. The samples were prepared on a copper plate with 10 mm in diameter. Three spots were measured on each sample.Electrochemical measurements
Electrochemical studies were carried out using Swagelok-type cells.29 In the case of MoS2/BC and S-MoS2/BC, the electrode materials were prepared by soaking the active materials in anhydrous 1-methyl-2-pyrrolidinone (NMP, Sigma-Aldrich, 99%). The slurry was pasted on a circular Cu plate (approx. 12 mm in diameter), dried overnight under vacuum at 75 °C and pressed. The resulting electrode was dried again in a vacuum oven at 75 °C for 24 h, and transferred into an Ar atmosphere glove box. The two-electrode Swagelok-type cell was assembled in the glove box using lithium foil as counter electrode and 1 M LiPF6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ethylene carbonate and dimethyl carbonate as the liquid electrolyte (Merck LP30). In contrast, the pure MoS2 nanosheet-based electrodes were prepared from a mixture of the active material, carbon black (SuperP, Timcal) and polyvinylidene fluoride (PVDF, Sigma-Aldrich, 99%) binder with a weight ratio of 8
1 mixture of ethylene carbonate and dimethyl carbonate as the liquid electrolyte (Merck LP30). In contrast, the pure MoS2 nanosheet-based electrodes were prepared from a mixture of the active material, carbon black (SuperP, Timcal) and polyvinylidene fluoride (PVDF, Sigma-Aldrich, 99%) binder with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, soaked in anhydrous NMP. The subsequent drying and pressing as well as the assembly process were the same as described above. Cyclic voltammetry and galvanostatic cycling of the cells were performed at 25 °C between 0.01 and 3.0 V versus Li+/Li at various scan/current rates using a VMP3 multichannel potentiostat (Bio-Logic SAS). Electrochemical impedance spectroscopy (EIS) was carried out also by using the VMP3 multichannel potentiostat in the frequency range of 100 kHz to 0.1 Hz.
1, soaked in anhydrous NMP. The subsequent drying and pressing as well as the assembly process were the same as described above. Cyclic voltammetry and galvanostatic cycling of the cells were performed at 25 °C between 0.01 and 3.0 V versus Li+/Li at various scan/current rates using a VMP3 multichannel potentiostat (Bio-Logic SAS). Electrochemical impedance spectroscopy (EIS) was carried out also by using the VMP3 multichannel potentiostat in the frequency range of 100 kHz to 0.1 Hz.
Results and discussion
The XRD patterns of pure MoS2, MoS2/BC and S-MoS2/BC are shown in Fig. 1. The diffraction peaks of the pure MoS2 sample (blue curve) match the hexagonal phase of MoS2 (space group P63/mmc, JCPDS card no. 37-1492).30 Following ref. 31, the detected peaks are assigned to the (002), (100) and (110) planes of MoS2, respectively. For MoS2/BC (red curve), the broad peak at around 20° corresponds to the BC matrix.28,32 The characteristic peaks of MoS2 are broad and of relatively low intensity which implies poor crystallinity of MoS2. In order to increase the crystallinity of MoS2, the composite was sintered at 700 °C for 10 h under Ar atmosphere. The XRD pattern of the sintered material S-MoS2/BC (black curve) indeed shows a sharp and pronounced (002) peak which is typical for well-stacked layers of MoS2 nanostructures.31 The peak position at 2θ = 14.25° corresponds to a d-spacing of 0.62 nm. As the peak width depends on the crystallinity and the strain, the (002) peak indicates a crystalline correlation length of the order of 5 nm, i.e. of about 8–10 ordered MoS2 layers. | ||
| Fig. 1 XRD patterns of S-MoS2/BC (black), MoS2/BC (red) and pure MoS2 nanosheets (blue). Circles indicate Bragg peaks associated to the hexagonal MoS2-phase (space group P63/mmc)31 while cubic indicated the diffraction peaks of BC. | ||
The surface chemical state and composition of the S-MoS2/BC composite were investigated by XPS analysis. The XPS survey spectrum in Fig. 2a confirms the presence of C, Mo, S, and O in the sample while the Cu signal originates from the substrate. In the high-resolution Mo 3d spectrum at binding energies of 223–235 eV (Fig. 2b), the peaks at 229.2 eV and 232.3 eV correspond to the 3d5/2 and 3d3/2 lines of Mo4+, respectively. The splitting is close to 3.1 eV, which is typical for Mo4+-ions.18,33 No Mo6+ signals are observed, indicating that no MoOx species are present in the composite. The high-resolution S 2p spectrum (Fig. 2c) exhibits peaks at 161.8 and 163.0 eV, which are associated with S2− 2p3/2 and 2p1/2. The intensity ratio of these two peaks is approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the separation energy is 1.2 eV, which is typical for S2−-ions. Quantitatively, the data (see Table S1†) imply a S
1 and the separation energy is 1.2 eV, which is typical for S2−-ions. Quantitatively, the data (see Table S1†) imply a S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo molar ratio of 2.24 ± 0.03, the small discrepancy to the stoichiometric ratio can be attributed to defects at the nanocomposite surfaces.
Mo molar ratio of 2.24 ± 0.03, the small discrepancy to the stoichiometric ratio can be attributed to defects at the nanocomposite surfaces.
 | ||
| Fig. 2 XPS spectra of the S-MoS2/BC composite: (a) survey scan and (b)–(e) high resolution scans of Mo 3d, S 2p, C 1s, and O 1s respectively. | ||
The C 1s spectrum in Fig. 2d shows an overlapped behavior with the main peak at 284 eV belonging to C–C bonds. The peak's right shoulder at 285.5 eV is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. In the O 1s spectrum in Fig. 2e, there are two split peaks. The one with lower binding energy attributes to the oxygen in the sintered nanofibers while the other belongs to oxygen adsorbed on the sample surface. The C to O ratio in S-MoS2/BC amounts to 3.35 ± 0.14 which clearly exceeds the C
O bonds. In the O 1s spectrum in Fig. 2e, there are two split peaks. The one with lower binding energy attributes to the oxygen in the sintered nanofibers while the other belongs to oxygen adsorbed on the sample surface. The C to O ratio in S-MoS2/BC amounts to 3.35 ± 0.14 which clearly exceeds the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O molar ratio in cellulose of 1.2. Considering the adsorbed oxygen, the C
O molar ratio in cellulose of 1.2. Considering the adsorbed oxygen, the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio in the actual material is supposed to be even higher. We conclude that the cellulose fibers matrix is partially carbonized and reduced during the sintering process. Finally, we note that the atomic composition studied at three different spots of S-MoS2/BC is quite similar, which demonstrates the uniform composition of the synthesized composite.
O ratio in the actual material is supposed to be even higher. We conclude that the cellulose fibers matrix is partially carbonized and reduced during the sintering process. Finally, we note that the atomic composition studied at three different spots of S-MoS2/BC is quite similar, which demonstrates the uniform composition of the synthesized composite.
SEM images of the MoS2/BC composite (Fig. 3) show that the composite displays a hierarchical structure. The pristine BC nanofibers display diameters of about 35 nm, forming a flexible, cross-linked three dimensional network (Fig. 3a). The hydrothermal synthesis step yields coating of the pristine BC nanofibers by MoS2 nanosheets as illustrated in Fig. 3b–d. The thickness of the MoS2 nanosheets is about 20–30 nm, which is in good agreement with the literature.30 Applying the same hydrothermal synthesis without the BC matrix, pure MoS2 nanosheets are formed which self-assemble into microspheres (Fig. S1†).
Appropriate sintering of the material at about 700–900 °C is required for obtaining MoS2 nanosheets with increased crystallinity. The thermal treatment should however not demolish the hierarchical structure. According to our previous study,34 the pristine BC matrix begins to decompose at around 380 °C. The decomposition temperature increases slightly if BC is combined with other materials which have a higher decomposition temperature.32 Here, we sintered the as-prepared MoS2/BC composite at 700 °C for 10 h under Ar atmosphere to carbonize the BC matrix and to boost the crystallinity of the MoS2 nanosheets. The microstructure of the sintered material S-MoS2/BC shown in Fig. 4 confirms that, under these conditions, the nanosheet-coated network of nanofibers is indeed preserved (cf. Fig. 3b–d).
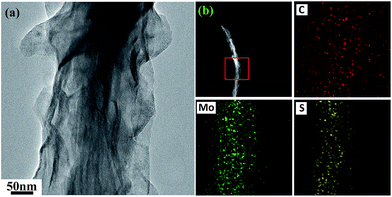
The morphology and the elemental distribution in the fibrous composites are further investigated by TEM and EDX. The dispersive MoS2 nanosheets on the fiber can be seen in the TEM image in Fig. 5a. The EDX elemental mapping in Fig. 5b clearly reveals that the Mo and S are evenly distributed in the fibers which C is found in the whole material. The EDX data clearly show that MoS2 is present at the fibers of the composite material.
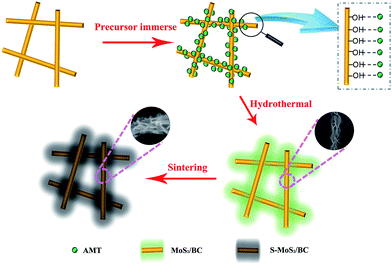
In the following, the strong regulation effect of BC is discussed. In general, bonding of the MoS2 nanosheets and the BC nanofibers is attributed to hydrogen bonds between –OH groups at the surfaces of the BC nanofibers and Mo7O246− anions from AMT. Because of the highly active surface of the BC fibers, the surface of BC is expected to adsorb large amounts of Mo precursor.12 MoS2 nanosheets are hence supposed to grow in situ on the surfaces of the BC nanofibers during the hydrothermal process. In order to prove these assumptions, several complementary experiments have been performed. Firstly, the Mo precursor was changed from AMT to MoS42−, keeping the same hydrothermal conditions. The microstructure of the resulting material is displayed in Fig. 6b. In contrast to the AMT-based synthesis (cf. Fig. 4), the microstructure reveals self-assembled MoS2-nanospheres appearing in the porous space in-between the BC nanofibers. These MoS2-nanospheres are smaller than those appearing in pure MoS2 obtained under the same hydrothermal conditions without BC (see Fig. S1†) which is attributed to the constricted space inside the fibrous network which restrains the nanospheres' further growth to microspheres. Except for this size effect, the morphology is very similar to that of pure MoS2, indicating that there is no further regulation factor of the BC matrix on MoS2 nanostructures when MoS42− is used as precursor. The different effect of the BC template during the synthesis can be associated to different bonding between the BC surfaces and the precursors. Compared with Mo7O246−, MoS42− exhibits weaker hydrogen bonds with the –OH groups so that MoS2 rarely grows at the BC surfaces. This result hence shows that strong hydrogen bonding is essential to modulate the growth of the MoS2 nanostructures. In the case of weak BC-precursor bonding, nucleation of MoS2 mostly happens in the interspace of the cross-linked BC nanofibrous network instead of at the surface of the nanofibers. Hence, the nanosheets tend to self-assemble to form spherical structures.
To further prove the relevance of hydrogen bonding at the BC surfaces, a modified BC matrix has been used during the hydrothermal reaction. This surface modification was achieved by treating the pristine 3D BC network with a 30% H2O2 solution overnight at room temperature. As a result, the surface of the BC nanofibers is expected to exhibit more oxygen containing groups but lower hydrogen content.18 Applying the original hydrothermal synthesis procedure with AMT but using the modified BC indeed yields clearly different results. After the reaction, only self-assembled MoS2 aggregates within the nanofibrous network are obtained (Fig. 6c).
The growth mechanism of the novel S-MoS2/BC architecture is summarized in Fig. 7. The BC matrix is first immersed into the Mo precursor solution. Mo7O246− anions from AMT form strong hydrogen bonds with the –OH groups of the BC nanofibers and disperse homogeneously on the surface of the nanofibers. This interaction further leads to nucleation of MoS2 on the surface of the nanofibers, ensuring MoS2 nanosheet growth on the nanofibers and preventing the agglomeration of the nanosheets into nanospheres. Abundance of hydrogen-containing surface groups (i.e. –OH groups) of the BC network is crucial for the uniform growth of MoS2 nanosheets on the nanofibers. The MoS2 nanosheets coated BC nanofiber structure is gradually formed upon hydrothermal treatment at appropriate temperature and pressure. Subsequent sintering of MoS2/BC at high temperature under protective atmosphere yields the highly crystallized MoS2 structure while the novel architecture is maintained.
To summarize, to the best of our knowledge this is the first example of a hierarchically structured nanocomposite organizing MoS2 nanosheets on a flexible 3D nanofiber network. Herein, the 3D BC structure offers a porous network, leaving adequate space for the access of electrolyte for the application in LIBs. In this case, the network of carbonized BC nanofibers is supposed to act as conductive nanowires. The 3D network may also prevent the aggregation of MoS2 nanosheets, endowing it with a high and stable specific surface area.
The electrochemical performance of the S-MoS2/BC composite was studied by means of cyclic voltammetry (CV), galvanostatic cycling (GCPL), and electrochemical impedance spectroscopy (EIS). The lithium storage mechanism of MoS2 is known to be strongly affected by the first lithiation half cycle, as the corresponding reduction products Mo and Li2S do not react back to the initial MoS2 phase. Instead, Li2S is oxidized to elemental sulfur during the delithiation, and S/Li2S form a redox couple during subsequent cycling. Based on ex situ XRD at different dis-/charge states, the following reaction steps have been reported:35,36
| 1st lithiation: MoS2 + xLi+ + xe− → LixMoS2 | (1a) |
| LixMoS2 + (4 − x)Li+ + (4 − x)e− → 2Li2S + Mo | (1b) |
| Subsequently: Li2S ↔ S + 2Li+ + 2e− | (2) |
Fig. 8 shows the 1st, 2nd, 6th, and 12th cycle of a CV, which was recorded at a scan rate of 0.1 mV s−1 in the voltage range of 0.01–3.0 V vs. Li/Li+. During the initial cathodic scan, there are two distinct reduction peaks around 1.0 V (Ia) and 0.5 V (Ib), and two further features around 1.6 V and 0.3 V. The first anodic scan shows one prominent oxidation peak at 2.25 V (ii), and two minor features around 1.7 V and 1.9 V, respectively. Reduction peak Ia indicates the intercalation of Li+-ions into the layered structure of MoS2 (eqn (1a)), while the ensuing conversion reaction (eqn (1b)) from LixMoS2 to Mo and Li2S is reflected in peak Ib.35 The reductive feature around 0.3 V, which is present in all cycles, in part can be attributed to the formation of a polymeric film on the active material due to electrolyte degradation.37–42 The prominent oxidation peak ii reflects the oxidation of Li2S to S (eqn (2)). This assignment is supported by a corresponding reductive feature (II) around 1.95 V during the 2nd cathodic scan. This feature evolves into a more pronounced double peak at 1.9/2.1 V upon further cycling, which is consistent with the multi-step conversion reaction of S8 to Li2S.43–45 Further evidence of the above-mentioned reaction mechanism (eqn (1) and (2)) is the strong decrease/disappearance of the reduction peaks Ia and Ib, respectively, after the 1st cycle. The fact that peak Ia persists in cycle 2, albeit with far less intensity, might indicate that the initial MoS2 phase was not converted completely during the 1st cycle. The additional redox activity, which occurs around 1.2–1.7 V/1.2–2.0 V during the cathodic and anodic scans, respectively, has not been elucidated thoroughly in literature yet, as the involved phases appear amorphous.36 The CV data (Fig. 8) indicate that reduction peak III at 1.45 V and oxidation peak iii around 1.8 V are related to each other, because both of them only emerge starting from the 2nd cycle on. Furthermore, oxidation peak iv, which evolves around 1.5 V, might be related to the reductive feature around 0.3 V, because both of them increase in intensity simultaneously from cycle 3 to 12. Possible mechanisms for these unknown redox reactions are the de-/lithiation of an amorphous Mo/Li2S matrix or amorphous MoSx; MoSx (e.g. MoS2) might form with excess S which could be present as the integrated intensity of the S reduction peak II is much smaller than the one of the corresponding oxidation ii.
Fig. 9 shows charge/discharge profiles of S-MoS2/BC during a GCPL at a C-rate of 1C (based on the MoS2 conversion reaction, i.e. 670 mA h g−1) in the potential range of 0.01–3.0 V vs. Li/Li+. During the initial charge cycle, two characteristic plateaus at 1.0 V and 0.6 V are observed which is consistent with the CV data (Fig. 8), and previous reports.46,47 The first plateau at 1.0 V corresponds to the intercalation of Li+ into the interlayer space of MoS2 (eqn (1a)). It is associated to an intake of 1.9 Li+/f.u. The next plateau at 0.6 V results from the conversion reaction (eqn (1b)) with about 3 Li+/f.u. intake. The composite exhibits an initial charge capacity of 1145 mA h g−1 at 1C. The charge profile changes considerably in the subsequent cycles, indicating the irreversible transformation of the active material, but remains rather stable from cycle 5 on. At this stage, the plateau-like features around 2.0/2.2 V during charge and discharge, respectively, indicate the formation of Li2S and its oxidation back to metallic S (eqn (2)).
The cycling performance, displayed in Fig. 10 for 50 cycles, is determined from GCPLs at 1C between 0.01 and 3 V. During the first 50 cycles, S-MoS2/BC shows high specific discharge capacities between 900 mA h g−1 and 1100 mA h g−1. Both the charge and discharge capacity do not fade during the first 40 cycles and even show a slight increase. This phenomenon occurs in many other kinds of conversion reaction materials such as Fe3O4 and CoO due to the reversible growth of a polymer/gel-like film caused by electrolyte decomposition, which leads to increased capacities during cycling.37,48 Moreover, the S-MoS2/BC composite exhibits a coulombic efficiency of >95% from the second cycle on. Compared with our pure MoS2 and non-sintered MoS2/BC samples, the S-MoS2/BC hierarchical structure shows enhanced specific capacities and improved cycling stability. The charge/discharge capacities of pure MoS2 begin to fade after the 1st cycle, decreasing to about 40% of the initial discharge capacity after 50 cycles. The cycling performance improves when BC nanofibers are used to form a MoS2/BC composite. However, its capacity is only stable during the first 15 cycles and begins to decrease afterwards. Sintering at 700 °C to obtain S-MoS2/BC with a carbonized nanofiber matrix further improves the cycling performance, as described above.
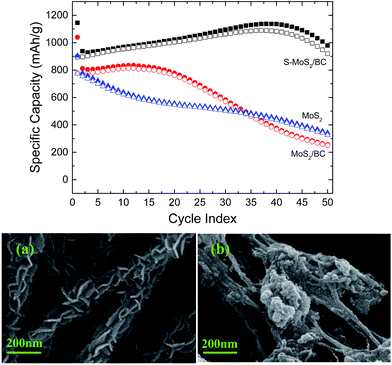 | ||
| Fig. 10 Top: Cycling performance of S-MoS2/BC, MoS2/BC and pure MoS2 at the C-rate of 1C. Bottom: SEM images of S-MoS2/BC before cycling (a) and after 50 charge–discharge cycles (b). | ||
The observed good initial cycling stability may be associated to the preserved fibrous morphology upon cycling and to the strong bonding between BC and MoS2 which is particularly enhanced by the final sintering step. This is evident from Fig. 10a and b, which show the microstructure of S-MoS2/BC before and after 50 charge/discharge cycles. The fibrous structure is still preserved after 50 cycles. However, there are pronounced changes regarding the active coating materials as the MoS2 nanosheets are altered to nanoparticles during the charge/discharge process. We conject that these changes lead to the capacity fade starting after 40 cycles. The long-term cycling studies of the S-MoS2/BC at the same current rate of 1C (see Fig. S2†) shows strong fading after 50 cycles.
The rate performance shown in Fig. 11 further illustrates the excellent electrochemical performance of S-MoS2/BC. The discharge capacity retains 1067 mA h g−1 after 5 cycles at a C-rate of 0.1C. When the current rate is increased to 0.2C, 0.5C, 1C, 2C, 5C and 10C, the corresponding discharge capacities are 1086 mA h g−1, 995 mA h g−1, 900 mA h g−1, 837 mA h g−1, 751 mA h g−1, 671 mA h g−1 after 5 cycles in each case, demonstrating high capacities and cycling stability even at high current densities. Finally, when the current rate is gradually decreased back to 0.1C, the discharge capacity recovers to 964 mA h g−1 which is almost the same as during the first 5 cycles at 0.1C, even though from the 45th cycle on the discharge capacities suffer from the degradation effect discussed by means of Fig. 10.
Electrochemical impedance spectra of pure MoS2, MoS2/BC and S-MoS2/BC obtained at frequencies between 100 kHz and 0.1 Hz provide further insight into the electrochemical processes. The Nyquist plots of all three samples before cycling, as shown in Fig. 12a, show a depressed semi-circle at high frequencies and a straight line at low ones. The semi-circles are described by means of a generalized RC-circuit with electrolyte resistance RE, charge transfer resistance RCT, and a constant phase element CPEDL for the electrical double layer, using the Z Fit function of the EC-Lab software (Bio-Logic). The used equivalent circuit is shown in Fig. 12a, and the calculated parameters are listed in Table S2.† The diameter of the semi-circle, which represents RCT, is similar for both the sintered and the non-sintered BC composites, while the pure MoS2 sample shows a significantly higher value. This indicates that the organic nanofiber network plays a crucial role, remarkably promoting the electron transfer in the composite electrode materials. The reduced resistance can be attributed to direct and efficient electrical pathways between the active material and the current collector, constructed by the conductive carbon nanofiber network. The results hence confirm that the electronic kinetics is increased strongly in the BC-based materials, which straightforwardly explains the significant enhancement of the cycling and rate performances in these materials. Comparatively, the RCT values of all three samples have decreased significantly after cycling (Fig. 12b). S-MoS2/BC has a lower resistance than MoS2/BC, indicating a more stable structure and better conductivity which explains the further enhanced cycling performance.49,50
 | ||
| Fig. 12 Nyquist plots of MoS2, MoS2/BC and S-MoS2/BC in the frequency range from 100 kHz to 0.1 Hz: (a) before cycling, (b) after 1 cycle. The insert of (a) shows the simulated circuit. | ||
In order to relate the electrochemical performance of the S-MoS2/BC nanocomposite to the literature, Table S3† lists the discharge capacities of various MoS2-based anode materials during the first 50 cycles. Based on these values, the S-MoS2/BC composite exhibits superior properties compared to other 3D hierarchical MoS2/carbon composites11,51 and MoS2-based binder-free electrodes.52,53 However, MoS2/CNT10 and MoS2/graphene54 composite materials usually show higher capacities and better cycling stability than the S-MoS2/BC counterpart. In contrast to these materials, the S-MoS2/BC composite benefits from the simple and well-controllable synthesis process, which includes inexpensive starting materials and a product which can be used as electrode material without further additives (viz. carbon black and binder).
Conclusions
In summary, we report the facile and cost-efficient synthesis of a hierarchically structured MoS2/C-nanocomposite in which MoS2-nanosheets are uniformly covering a flexible 3D nanofiber network of carbonized bacterial cellulose. The nanocomposite is used as a binder-free and carbon-free anode material of LIBs. It exhibits enhanced lithium storage properties including a high initial capacity (1137 mA h g−1 at 1C), good cycling stability (967 mA h g−1 after 50 cycles at 1C) and excellent rate performance. Such outstanding performance can be ascribed to the advantages endowed by the porous 3D network structure: tight connection between MoS2 and nanofibers, and a highly conductive matrix. The strong connections between the active material and the matrix, which was derived from the hydrogen bonding of the precursors, help to buffer the volume changes upon electrochemical cycling and increase the electron kinetics, playing a key role herein. The strategy to employ low-cost, environmentally friendly, flexible, 3D web-like structure offers an effective and versatile way to design novel binder-free carbon-free electrode materials. In addition to the excellent performance in lithium ion batteries, the S-MoS2/BC with unique structure is also a good candidate for other applications demanding stable, nanoporous, and conductive MoS2/C-composites such as hydrogen evolution and photocatalysis.Acknowledgements
The authors thank I. Glass for technical support. Z. L. acknowledges financial support by the Chinese Scholarship Council and by the Götze foundation. The authors are grateful for financial support of the CleanTech-Initiative of the Baden-Württemberg-Stiftung (Project CT3 Nanostorage). A. O. and C. N. acknowledge support by the IMPRS-QD and the Heidelberg Graduate School of Fundamental Physics (HGSFP), respectively.References
- S. Ding, D. Zhang, J. S. Chen and X. W. Lou, Nanoscale, 2012, 4, 95–98 RSC.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- Q. He, Z. Zeng, Z. Yin, H. Li, S. Wu, X. Huang and H. Zhang, Small, 2012, 8, 2994–2999 CrossRef CAS PubMed.
- G. Yue, J. Wu, Y. Xiao, M. Huang, J. Lin and J.-Y. Lin, J. Mater. Chem. A, 2013, 1, 1495–1501 CAS.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- K. F. Mak, K. He, J. Shan and T. F. Heinz, Nat. Nanotechnol., 2012, 7, 494–498 CrossRef CAS PubMed.
- R. Suzuki, M. Sakano, Y. J. Zhang, R. Akashi, D. Morikawa, A. Harasawa, K. Yaji, K. Kuroda, K. Miyamoto, T. Okuda, K. Ishizaka, R. Arita and Y. Iwasa, Nat. Nanotechnol., 2014, 9, 611–617 CrossRef CAS PubMed.
- X. Hong, J. Kim, S.-F. Shi, Y. Zhang, C. Jin, Y. Sun, S. Tongay, J. Wu, Y. Zhang and F. Wang, Nat. Nanotechnol., 2014, 9, 682–686 CrossRef CAS PubMed.
- C. Zhang, Z. Wang, Z. Guo and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3765–3768 CAS.
- C. Lu, W.-w. Liu, H. Li and B. K. Tay, Chem. Commun., 2014, 50, 3338–3340 RSC.
- H. Yu, C. Zhu, K. Zhang, Y. Chen, C. Li, P. Gao, P. Yang and Q. Ouyang, J. Mater. Chem. A, 2014, 2, 4551–4557 CAS.
- Z. Wan, J. Shao, J. Yun, H. Zheng, T. Gao, M. Shen, Q. Qu and H. Zheng, Small, 2014, 10, 4975–4981 CrossRef CAS PubMed.
- J. Park, J.-S. Kim, J.-W. Park, T.-H. Nam, K.-W. Kim, J.-H. Ahn, G. Wang and H.-J. Ahn, Electrochim. Acta, 2013, 92, 427–432 CrossRef CAS.
- K. Chang and W. Chen, ACS Nano, 2011, 5, 4720–4728 CrossRef CAS PubMed.
- J. S. Cho, Y. J. Hong and Y. C. Kang, ACS Nano, 2015, 9, 4026–4035 CrossRef CAS PubMed.
- M. Wang, G. Li, H. Xu, Y. Qian and J. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 1003–1008 CAS.
- H. Yoo, A. P. Tiwari, J. Lee, D. Kim, J. H. Park and H. Lee, Nanoscale, 2015, 7, 3404–3409 RSC.
- S. Zhang, X. Yu, H. Yu, Y. Chen, P. Gao, C. Li and C. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 21880–21885 CAS.
- X. Zhou, Z. Wang, W. Chen, L. Ma, D. Chen and J. Y. Lee, J. Power Sources, 2014, 251, 264–268 CrossRef CAS.
- Y. Gong, S. Yang, Z. Liu, L. Ma, R. Vajtai and P. M. Ajayan, Adv. Mater., 2013, 25, 3979–3984 CrossRef CAS PubMed.
- G. Huang, T. Chen, W. Chen, Z. Wang, K. Chang, L. Ma, F. Huang, D. Chen and J. Y. Lee, Small, 2013, 9, 3693–3703 CrossRef CAS PubMed.
- C. Wu, J. Maier and Y. Yu, Adv. Mater., 2016, 28, 174–180 CrossRef CAS PubMed.
- X. Zhou, L.-J. Wan and Y.-G. Guo, Nanoscale, 2012, 4, 5868–5871 RSC.
- H. Li and X. Wang, Sci. China: Chem., 2015, 58, 1792–1799 CrossRef CAS.
- H.-X. Zhang, C. Feng, Y.-C. Zhai, K.-L. Jiang, Q.-Q. Li and S.-S. Fan, Adv. Mater., 2009, 21, 2299–2304 CrossRef CAS.
- I. Lahiri, S.-W. Oh, J. Y. Hwang, S. Cho, Y.-K. Sun, R. Banerjee and W. Choi, ACS Nano, 2010, 4, 3440–3446 CrossRef CAS PubMed.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Energy Environ. Sci., 2014, 7, 209–231 CAS.
- H. Sai, L. Xing, J. Xiang, L. Cui, J. Jiao, C. Zhao, Z. Li and F. Li, J. Mater. Chem. A, 2013, 1, 7963–7970 CAS.
- C. Neef, C. Jahne, H. P. Meyer and R. Klingeler, Langmuir, 2013, 29, 8054–8060 CrossRef CAS PubMed.
- U. K. Sen and S. Mitra, ACS Appl. Mater. Interfaces, 2013, 5, 1240–1247 CAS.
- M. Chhowalla and G. A. J. Amaratunga, Nature, 2000, 407, 164–167 CrossRef CAS PubMed.
- H. Sai, L. Xing, J. Xiang, L. Cui, J. Jiao, C. Zhao, Z. Li, F. Li and T. Zhang, RSC Adv., 2014, 4, 30453–30461 RSC.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- H. Sai, R. Fu, L. Xing, J. Xiang, Z. Li, F. Li and T. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 7373–7381 CAS.
- J. Xiao, X. Wang, X.-Q. Yang, S. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21, 2840–2846 CrossRef CAS.
- X. Fang, X. Guo, Y. Mao, C. Hua, L. Shen, Y. Hu, Z. Wang, F. Wu and L. Chen, Chem.–Asian J., 2012, 7, 1013–1017 CrossRef CAS PubMed.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dollé, L. Dupont and J.-M. Tarascon, J. Electrochem. Soc., 2002, 149, A627–A634 CrossRef CAS.
- Q. Wang and J. Li, J. Phys. Chem. C, 2007, 111, 1675–1682 CAS.
- J.-M. Tarascon, S. Grugeon, M. Morcrette, S. Laruelle, P. Rozier and P. Poizot, C. R. Chim., 2005, 8, 9–15 CrossRef CAS.
- S. Grugeon, S. Laruelle, L. Dupont and J. M. Tarascon, Solid State Sci., 2003, 5, 895–904 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon and J.-M. Tarascon, J. Electrochem. Soc., 2002, 149, A1212–A1217 CrossRef CAS.
- A. Débart, L. Dupont, R. Patrice and J. M. Tarascon, Solid State Sci., 2006, 8, 640–651 CrossRef.
- P. Leghié, J. P. Lelieur and E. Levillain, Electrochem. Commun., 2002, 4, 406–411 CrossRef.
- J. Wang, J. Yang, C. Wan, K. Du, J. Xie and N. Xu, Adv. Funct. Mater., 2003, 13, 487–492 CrossRef CAS.
- S.-E. Cheon, K.-S. Ko, J.-H. Cho, S.-W. Kim, E.-Y. Chin and H.-T. Kim, J. Electrochem. Soc., 2003, 150, A796–A799 CrossRef CAS.
- H. Yoo, A. P. Tiwari, J.-T. Lee, D. Kim, J. H. Park and H. Lee, Nanoscale, 2015, 7(8), 3404–3409 RSC.
- L. Zhang, H. B. Wu, Y. Yan, X. Wang and X. W. Lou, Energy Environ. Sci., 2014, 7, 3302–3306 CAS.
- C. Ban, Z. Wu, D. T. Gillaspie, L. Chen, Y. Yan, J. L. Blackburn and A. C. Dillon, Adv. Mater., 2010, 22, E145–E149 CrossRef CAS PubMed.
- J. S. Cho, H. S. Ju and Y. C. Kang, Sci. Rep., 2016, 6, 23915 CrossRef CAS PubMed.
- Y. J. Hong and Y. C. Kang, Small, 2015, 11, 2157–2163 CrossRef CAS PubMed.
- Y.-E. Miao, Y. Huang, L. Zhang, W. Fan, F. Lai and T. Liu, Nanoscale, 2015, 7, 11093–11101 RSC.
- X. Cao, Y. Shi, W. Shi, X. Rui, Q. Yan, J. Kong and H. Zhang, Small, 2013, 9, 3433–3438 CrossRef CAS PubMed.
- C. Wang, W. Wan, Y. Huang, J. Chen, H. H. Zhou and X. X. Zhang, Nanoscale, 2014, 6, 5351–5358 CAS.
- X. Zhou, L.-J. Wan and Y.-G. Guo, Chem. Commun., 2013, 49, 1838–1840 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11214e |
| This journal is © The Royal Society of Chemistry 2016 |