An all-solid-state imprinted polymer-based potentiometric sensor for determination of bisphenol S†
Abstract
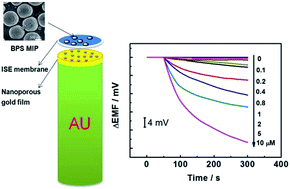
An all-solid-state polymeric membrane potentiometric sensor for determination of bisphenol S has been developed by using the imprinted polymer as the receptor and a nanoporous gold film as the solid contact. The sensor has a linear concentration range of 0.1 to 2 μM with a detection limit of 0.04 μM.

 Please wait while we load your content...
Please wait while we load your content...