Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 4-thio-5-(2′′-thienyl)uridine and cytotoxicity activity against colon cancer cells in vitro†
Xiaohui
Zhang
a,
Depeng
Li
a,
Jianzhong
Qin
b,
Yaozhong
Xu
*c and
Kedong
Ma
*a
aCollege of Environment and Chemical Engineering, Dalian University, Dalian 116622, PR China. E-mail: makedongdl@yahoo.co.jp; Fax: +86-411-87402440; Tel: +86-411-87402440
bSchool of Life Science and Biotechnology, Dalian University, Dalian 116622, China
cDepartment of Life, Health and Chemical Sciences, The Open University, Walton Hall, Milton Keynes, MK7 6AA, UK. E-mail: y.z.xu@open.ac.uk
First published on 19th July 2016
Abstract
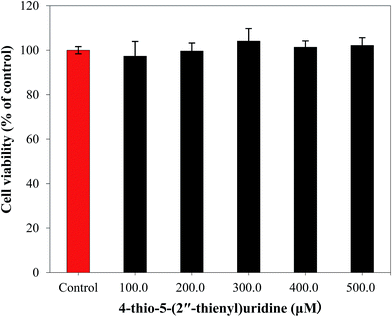
A novel anti-tumor agent 4-thio-5-(2′′-thienyl)uridine (6) was synthesized and the in vitro cytotoxicity activity against mice colon cancer cells (MC-38) and human colon cancer cells (HT-29) was evaluated by MTT assay. The results showed that the novel compound had antiproliferative activity toward MC-38 and HT-29 cells in a dose-dependent manner. The cell cycle analysis by flow cytometry indicated that compound 6 exerted in tumor cell proliferation inhibition by arresting HT-29 cells in the G2/M phase. In addition, cell death detected by propidium iodide staining showed that compound 6 efficiently induced cell apoptosis in a concentration-dependent manner. Moreover, the sensitivity of human fibroblast cells to compound 6 was far lower than that of tumor cells, suggesting the specific anti-tumor effect of 4-thio-5-(2′′-thienyl)uridine. Taken together, novel compound 6 effectively inhibits colon cancer cell proliferation, and hence would have potential value in clinical application as an antitumor agent.
1. Introduction
The flow of genetic information is directed by nucleic acids (DNA and RNA). The base analogues structurally similar to the four canonical bases can be incorporated into DNA, which alter their properties and facilitate basic studies of DNA-related processes such as its interaction with proteins, replication and transcription.1,2 Structural modification of nucleosides is biologically meaningful, and has led to the discovery of a variety of new therapeutic agents, which includes antiviral and anticancer agents. Pyrimidines and fused pyrimidines, being an integral part of DNA and RNA have considerable chemical and pharmacological importance. Thio-pyrimidine compounds in which an oxygen atom in the base is replaced by sulfur have been reported to show improved anti-cancer activity and immune enhancement.3–7Thiophene and its derivatives as important sulfur-containing five-member heterocyclic compounds have various biological and pharmacological properties in terms of antimicrobial and antitumor activities.8–10 As a rich electronic aromatic ring compound, the charge density of thiophene is greater than that of benzene ring, and thus, it is easy for thiophene to occur the π–π interactions. Moreover, the sulfur atom in thiophene is certain nucleophilic and easy to interact with electrophilic receptors or form metal or metal ion. From the viewpoint of reducing the drug side effects, enhancing the drug targeting and improving the pharmacokinetic properties of drugs and the corresponding therapeutic index, new compound with the characteristics of both thiophene and thio-pyrimidine are certainly with explore as attractive antitumor agent.11,12
Colon cancer is a kind of digestive tract malignant tumor, commonly occurring at the junction of the rectum and sigmoid colon. The incidence of colon cancer begins to rise at age 40 and peaks at age 60 to 75, and the main life-style related cause is high-fat diet and inadequate cellulose intake. Colon cancer ranks third among the leading causes of cancer-associated death after lung and prostate cancer for men and after lung and breast cancer for women.13 In recent years, there is an upward trend in China as the improvement of people's living standard and changes in diet. Accordingly, the development of novel chemotherapeutic agents is an urgent issue.
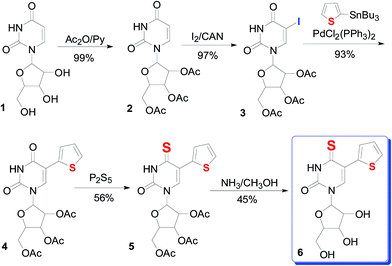
In this study, a novel 4-thio-5-(2′′-thienyl)uridine (6) compound was synthesized by introducing a thiophene to uridine by a series of reaction (Scheme 1). The cytotoxicity of compound was evaluated by in vitro administering to mice colon cancer cells line (MC-38) and human colon cancer cells line (HT-29) by MTT assay, respectively. Compound 6 was found to have antiproliferative activity against colon cancer cells. Flow cytometry examination revealed that compound 6 inhibited cell growth through cell cycle arrest at G2/M phase. Combined with no cytotoxic and apoptotic effect on human fibroblast cells, 4-thio-5-(2′′-thienyl)uridine (6) have potential clinical applications.
2. Experimental section
2.1. Apparatus and materials
Reagents were obtained from commercial suppliers and were used without further purification. Melting point was determined on a XR-4-type micro-melting point detector, and was uncorrected. The compounds synthesized were purified by column chromatography using silica gel (200–300 mesh) except for recrystallization and thin-layer chromatography (TLC) using silica gel 60 F254 plates (250 mm; Qingdao Ocean Chemical Company, China). IR spectra were recorded using a Nicolet 550 Spectrophotometer (4000–400 cm−1) with a crystalline sample spread on KBr pellets. UV spectra were recorded using UV-vis spectrophotometer (JASCO, Japan); 1H NMR and 13C NMR spectra were obtained by a 500 MHz Bruker AV-400 spectrometer with TMS as an internal standard. The mass spectrum was obtained on Hewlett-Packard 1100 LC/MSD spectrometer.2.2. Crystal structure
The crystallographic data for 4-thio-5-(2′′-thienyl)uridine were summarized in Table 1. X-ray diffraction data were collected at 120 K using a Bruker Nonius Kappa CCD diffractometer mounted at the window of a Bruker FR591 rotating anode (Mo Kα, λ = 0.71073 Å) and equipped with Bruker APEX II detector and Oxford Cryosystems Cryostream device. ORTEP views of 4-thio-5-(2′′-thienyl)uridine were given in Fig. S4.† The structure was solved by direct methods using SHELXS97.22 The crystal structure has been deposited in Cambridge Structural Database: CCDC depository number 1479957.| Compound | 4-thio-5-(2′′-thienyl)uridine |
| Empirical formula | C13H15N2O5.50S2 |
| Formula weight | 351.39 |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell dimensions | a = 14.5426(10) Å, α = 90° |
| b = 7.8358(5) Å, β = 98.267(2)° | |
| c = 50.555(4) Å, γ = 90° | |
| Volume | 5701.4(7) Å3 |
| Z | 16 |
| Density (calculated) | 1.637 mg m−3 |
| Absorption coefficient | 0.404 mm−1 |
| F(000) | 2928 |
| Crystal | Cut blade; yellow |
| Crystal size | 0.170 × 0.090 × 0.030 mm3 |
| θ range for data collection | 2.306–27.512° |
| Index ranges | −18 ≤ h ≤ 18, −10 ≤ k ≤ 10, −64 ≤ l ≤ 65 |
| Reflections collected | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 998 998 |
| Independent reflections | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 [Rint = 0.0589] 854 [Rint = 0.0589] |
| Goodness-of-fit on F2 | 1.031 |
| Final R indices [F2 > 2σ(F2)] | R 1 = 0.0469, wR2 = 0.1208 |
| R indices (all data) | R 1 = 0.0562, wR2 = 0.1265 |
| Absolute structure parameter | 0.02(3) |
| Extinction coefficient | n/a |
| Largest diff. peak and hole | 0.775 and −0.402 e Å−3 |
2.3. Synthesis and characterization of 4-thio-5-(2′′-thienyl)uridine and its intermediates (1–6)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3); IR (film)/cm−1: 1755, 1720 (C
OCH3); IR (film)/cm−1: 1755, 1720 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1694 (amide C
O), 1694 (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1630 (C
O), 1630 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).
C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solvent was evaporated and the residue was partitioned in a cold mixture of EtOAc (20 mL), saturated NaCl/H2O (10 mL), NaHSO3/H2O (5 mL). Then, the aqueous layer was extracted with EtOAc (10 mL × 2). The organic layer was collected and washed carefully using 5% cold NaHSO3/H2O (5 mL), then followed by saturated NaCl/H2O (15 mL) and H2O (15 mL × 2), finally dried by anhydrous magnesium sulfate to remove water. The crude 5-iodo products were purified by column chromatography or recrystallized to give the target compound (3) with the yield around 97%, mp: 175–176 °C (lit. mp: 177–178 °C). 1H NMR (400 MHz, DMSO-d6) δ: 11.83 (brs, 1H, NH), 8.18 (s, 1H, 6-H), 5.88 (d, J = 4.0 Hz, 1H, 1′-H), 5.47 (dd, J = 8.0 Hz, 1H, 2′-H), 5.33–5.35 (m, 1H, 3′-H), 4.31–4.36 (m, 1H, 4′-H), 4.21–4.27 (m, 2H, 5′-H), 2.06, 2.07, 2.08 (3 s, 3H, 3H, 3H, OAc); UV (in CH3CN): λmin = 257.0 nm, 226.5 nm.
1). The solvent was evaporated and the residue was partitioned in a cold mixture of EtOAc (20 mL), saturated NaCl/H2O (10 mL), NaHSO3/H2O (5 mL). Then, the aqueous layer was extracted with EtOAc (10 mL × 2). The organic layer was collected and washed carefully using 5% cold NaHSO3/H2O (5 mL), then followed by saturated NaCl/H2O (15 mL) and H2O (15 mL × 2), finally dried by anhydrous magnesium sulfate to remove water. The crude 5-iodo products were purified by column chromatography or recrystallized to give the target compound (3) with the yield around 97%, mp: 175–176 °C (lit. mp: 177–178 °C). 1H NMR (400 MHz, DMSO-d6) δ: 11.83 (brs, 1H, NH), 8.18 (s, 1H, 6-H), 5.88 (d, J = 4.0 Hz, 1H, 1′-H), 5.47 (dd, J = 8.0 Hz, 1H, 2′-H), 5.33–5.35 (m, 1H, 3′-H), 4.31–4.36 (m, 1H, 4′-H), 4.21–4.27 (m, 2H, 5′-H), 2.06, 2.07, 2.08 (3 s, 3H, 3H, 3H, OAc); UV (in CH3CN): λmin = 257.0 nm, 226.5 nm.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 137.27 (C-4), 126.92 (C-2), 126.71 (C-2′′), 124.02 (C-5′′), 109.51 (C-6), 89.28 (C-4′′), 79.75 (C-3′′), 79.64 (C-5), 72.51 (C-4′), 72.44 (C-1′), 70.07 (C-3′), 69.95 (C-5′), 63.44 (C-2′), 20.97, 20.77, 20.71 (3×–CH3); HRMS: [M + Na]+ 475.0790, calculated for C19H20N2O9SNa; found 475.0793. UV-vis (in CH3CN): λmax/nm: 262.0, 304.0. IR (film)/cm−1: 1748.0 (C
O), 137.27 (C-4), 126.92 (C-2), 126.71 (C-2′′), 124.02 (C-5′′), 109.51 (C-6), 89.28 (C-4′′), 79.75 (C-3′′), 79.64 (C-5), 72.51 (C-4′), 72.44 (C-1′), 70.07 (C-3′), 69.95 (C-5′), 63.44 (C-2′), 20.97, 20.77, 20.71 (3×–CH3); HRMS: [M + Na]+ 475.0790, calculated for C19H20N2O9SNa; found 475.0793. UV-vis (in CH3CN): λmax/nm: 262.0, 304.0. IR (film)/cm−1: 1748.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3), 1717.5 (C
OCH3), 1717.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1459.4, 1374.3 (NH), 1571.3, 1414.0, 1299.8 (C
O), 1459.4, 1374.3 (NH), 1571.3, 1414.0, 1299.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1627.47 (C5
C), 1627.47 (C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6).
C6).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) to give the title compound (5) at yield of 56%. Mp: 171–173 °C. 1H NMR (400 MHz, DMSO-d6) δ: 13.07 (brs, 1H, NH), 8.05 (s, 1H, 6-H), 7.57–7.58 (d, 1H, J = 4.0 Hz, 5′′-H), 7.29–7.30 (d, 1H, J = 4.0 Hz, 3′′-H), 7.06–7.08 (t, 1H, J = 4.0 Hz, 3′-H), 5.35–5.41 (m, 1H, 2′-H), 4.25–4.36 (m, 3H, 4′-H, 5′-H), 2.07 (m, 9H, CH3); 13C NMR (DMSO-d6, 500 MHz) δ: 188.58 (C-4), 170.43, 169.78, 169.75 (3×–C
2, v/v) to give the title compound (5) at yield of 56%. Mp: 171–173 °C. 1H NMR (400 MHz, DMSO-d6) δ: 13.07 (brs, 1H, NH), 8.05 (s, 1H, 6-H), 7.57–7.58 (d, 1H, J = 4.0 Hz, 5′′-H), 7.29–7.30 (d, 1H, J = 4.0 Hz, 3′′-H), 7.06–7.08 (t, 1H, J = 4.0 Hz, 3′-H), 5.35–5.41 (m, 1H, 2′-H), 4.25–4.36 (m, 3H, 4′-H, 5′-H), 2.07 (m, 9H, CH3); 13C NMR (DMSO-d6, 500 MHz) δ: 188.58 (C-4), 170.43, 169.78, 169.75 (3×–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 147.07 (C-2), 136.74 (C-2′′), 136.41 (C-5′′), 127.11 (C-6), 126.34 (C-4′′), 117.96 (C-3′′), 89.96 (C-5), 79.97 (C-4′), 72.72 (C-1′), 69.92 (C-3′), 66.83 (C-5′), 63.22 (C-2′), 20.81, 20.77, 20.74 (3×–CH3); HRMS: [M + H]+ 467.0741, calculated for C19H20N2O8S2; found 469.0727. UV-vis (in CH3CN): λmax/nm: 239, 285, 348. IR (film)/cm−1: 1747.5 (C
O), 147.07 (C-2), 136.74 (C-2′′), 136.41 (C-5′′), 127.11 (C-6), 126.34 (C-4′′), 117.96 (C-3′′), 89.96 (C-5), 79.97 (C-4′), 72.72 (C-1′), 69.92 (C-3′), 66.83 (C-5′), 63.22 (C-2′), 20.81, 20.77, 20.74 (3×–CH3); HRMS: [M + H]+ 467.0741, calculated for C19H20N2O8S2; found 469.0727. UV-vis (in CH3CN): λmax/nm: 239, 285, 348. IR (film)/cm−1: 1747.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3), 1715.6 (C
OCH3), 1715.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1451.7, 1371.3 (NH), 1522.7, 1227.5, 1427.31 (C
O), 1451.7, 1371.3 (NH), 1522.7, 1227.5, 1427.31 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1610 (C5
C), 1610 (C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6), 1108 (C
C6), 1108 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S).
S).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 9
MeOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and provide a solid product at yields of 45%, namely 4-thio-5-(2′′-thienyl) nucleoside (6). Mp: 185–187 °C. 1H NMR (400 MHz, DMSO-d6) δ: 12.89 (brs, 1H, NH), 8.51 (s, 1H, 6-H), 7.47–7.48 (d, 1H, J = 4.0 Hz, 5′′-H), 7.27–7.28 (m, 1H, 3′′-H), 6.98–7.00 (m, 1H, 4′′-H), 5.75–5.76 (d, 1H, J = 4.0 Hz, 1′-H), 5.50–5.51 (d, 1H, J = 4.0 Hz, OH), 5.30–5.32 (t, 1H, J = 4.0 Hz, OH), 5.06–5.07 (d, 1H, J = 4.0 Hz, 5′-OH), 4.10–4.14 (m, 1H, 3′-H), 4.00–4.04 (m, 1H, 2′-H), 3.89–3.90 (m, 1H, 4′-H), 3.56–3.72 (m, 2H, 5′-H); 13C NMR (DMSO-d6, 500 MHz) δ: 187.52 (C-4), 172.04 (C-2), 147.36 (C-2′′), 136.91 (C-5′′), 135.73 (C-6), 127.09 (C-4′′), 126.23 (C-3′′), 117.61 (C-5), 89.80(C-4′), 85.20 (C-1′), 74.87 (C-3′), 69.57 (C-5′), 60.32 (C-2′), HRMS: [M + Na]+ 365.0244, calculated for C13H14N2O5S2Na; found 365.0249. UV-vis (in CH3CN): λmax/nm: 239, 287, 356. IR (film)/cm−1: 3344.3 (–OH), 1699.7 (C
1, v/v) and provide a solid product at yields of 45%, namely 4-thio-5-(2′′-thienyl) nucleoside (6). Mp: 185–187 °C. 1H NMR (400 MHz, DMSO-d6) δ: 12.89 (brs, 1H, NH), 8.51 (s, 1H, 6-H), 7.47–7.48 (d, 1H, J = 4.0 Hz, 5′′-H), 7.27–7.28 (m, 1H, 3′′-H), 6.98–7.00 (m, 1H, 4′′-H), 5.75–5.76 (d, 1H, J = 4.0 Hz, 1′-H), 5.50–5.51 (d, 1H, J = 4.0 Hz, OH), 5.30–5.32 (t, 1H, J = 4.0 Hz, OH), 5.06–5.07 (d, 1H, J = 4.0 Hz, 5′-OH), 4.10–4.14 (m, 1H, 3′-H), 4.00–4.04 (m, 1H, 2′-H), 3.89–3.90 (m, 1H, 4′-H), 3.56–3.72 (m, 2H, 5′-H); 13C NMR (DMSO-d6, 500 MHz) δ: 187.52 (C-4), 172.04 (C-2), 147.36 (C-2′′), 136.91 (C-5′′), 135.73 (C-6), 127.09 (C-4′′), 126.23 (C-3′′), 117.61 (C-5), 89.80(C-4′), 85.20 (C-1′), 74.87 (C-3′), 69.57 (C-5′), 60.32 (C-2′), HRMS: [M + Na]+ 365.0244, calculated for C13H14N2O5S2Na; found 365.0249. UV-vis (in CH3CN): λmax/nm: 239, 287, 356. IR (film)/cm−1: 3344.3 (–OH), 1699.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1519.0, 1280.8 (C
O), 1519.0, 1280.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1600.2 (C5
C), 1600.2 (C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6), 1122.6 (C
C6), 1122.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S).
S).
2.4. Toxicity test on colon cancer cells
The MTT colorimetric method and flow cytometry were employed to analyze the anti-tumor activity of 4-thio-5-(2′′-thienyl)uridine on mice colon cancer cell line MC-38 and human colon cancer cell line HT-29, which were purchased from the American Type Culture Collection.2.5. Statistical analysis
Data were presented as mean ± SD of three independent experiments. One-way analysis of variance (ANOVA) was performed on the data to assess the impact of the variables on the results. SPSS 13.0 statistical software (SPSS Inc., Chicago, USA) was used for the statistical analysis of all data. A p value of ≤0.05 was considered to be statistically significant.3. Results and discussion
3.1. Chemistry synthesis
3.2. Cytotoxicity activities
 | ||
| Fig. 3 Effects of different concentrations of compound 6 on cell cycle distribution of HT-29. The experiment was repeated thrice. Here only representative flow cytometric graphs are shown. | ||
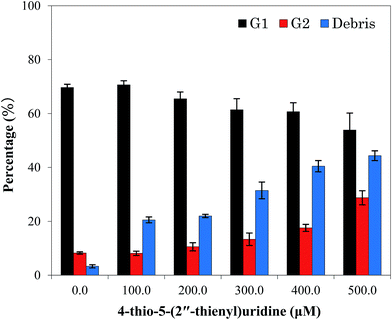 | ||
| Fig. 4 Quantitative data of cell cycle analysis of HT-29 treated with different concentrations of compound 6 for 24 h. Each value represents the mean ± SD of three independent experiments. | ||
In addition, a fraction of cell population with DNA content lower than that of G1 (sub-G1 phase) gradually increased after exposure to compound 6, from 10.3% (control group) to 20.54% (100 μM), 21.197% (200 μM), 31.47% (300 μM), 40.46% (400 μM), and 44.63% (500 μM), respectively. As the DNA replication had not yet occurred at G1 phase, the total DNA content lower than the value in G1 phase indicated the occurrence of DNA degradation in the cell, which commonly was considered as an important feature of cell apoptosis.20,21 Therefore, the increase of cell numbers at the sub-G1 phase revealed that compound 6 could induce apoptosis of HT-29 cell at such low concentration of 100 μM. Meanwhile, the population of apoptotic cells elevated under higher concentration, suggesting the apoptosis induction occurred in a dose-dependent manner. These results indicated that cell cycle arrest in the G2/M stage and apoptosis induction was important factors by which compound 6 exerted its inhibitory effects on HT-29 cells.
4. Conclusions
In this study, we successfully synthesized a novel compound: 4-thio-5-(2′′-thienyl)uridine (6) by connecting thiophene to 5-position of thiopyrimidine. In vitro pharmacological analysis demonstrated the compound 6 exerted proliferation inhibitory activity against MC-38 and HT-29 cells by arresting cell cycle at the G2/M phase and inducing apoptosis in a dose-dependent manner. The sensitivity of the normal human fibroblasts to compound 6 was found to be substantially lower than that of the tumor cells and different concentrations showed no significant inhibition of normal fibroblast proliferation, suggesting that 4-thio-5-(2′′-thienyl)uridine (6) and its analogues would be promising candidates for anti-tumor drug development.Acknowledgements
The work was supported by the Dalian government's science-technology plan projects (Grant No. 2014E12SF074), the public welfare foundation project of science enterprise investigation in Liaoning province of china (2015001024), the Dalian Jinzhou New Area government's science-technology plan projects (Grant No. KJCX-ZTPY-2014-0009) and Liaoning Province Education Administration (Grant No. L2013472).Notes and references
- I. V. Kutyavin, R. L. Rhinehart, E. A. Lukhtanov, V. V. Gorn, R. B. Meyer and H. B. Gamper, Biochemistry, 1996, 35, 11170 CrossRef CAS PubMed.
- A. M. Sismour and S. A. Benner, Nucleic Acids Res., 2005, 33, 5640 CrossRef CAS PubMed.
- S. C. Nigmn, G. S. Sahara and H. R. Shamm, J. Indian Chem. Soc., 1983, 60, 583 Search PubMed.
- A. B. Sen and R. N. Kapoor, J. Indian Chem. Soc., 1973, 50, 486 CAS.
- A. G. Lezius and K. H. Scheit, Eur. J. Biochem., 1967, 3, 85 CrossRef CAS PubMed.
- M. Sprinzl, K. H. Scheit and F. Cramer, Eur. J. Biochem., 1973, 34, 306 CrossRef CAS PubMed.
- R. S. Coleman and E. A. Kesicki, J. Am. Chem. Soc., 1994, 116, 11636 CrossRef CAS.
- B. Bertoša, M. Aleksić, G. Karminiski-Zamola and S. Tomić, Int. J. Pharm., 2010, 394, 106 CrossRef PubMed.
- Y. M. Lin, Y. Zhou, M. T. Flavin, L. M. Zhou, W. Nie and F. C. Chen, Bioorg. Med. Chem., 2002, 10, 2795 CrossRef CAS PubMed.
- H. M. Gabar and M. C. Bagley, Eur. J. Chem., 2011, 2, 214 CrossRef.
- Y. Tang, J. Zhang, S. Zhang, R. Geng and C. Zhou, Chin. J. Chem., 2012, 30, 1831 CrossRef CAS.
- Y. Tang, Master thesis, Southwest University, Chongqing, P.R. China, 2012.
- National Comprehensive Cancer Network, NCCN clinical practice guidelines in oncology, colon cancer, V3, 2013 Search PubMed.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508 CrossRef.
- P. Wigerinck, L. Kerremans, P. Claes, R. Snoeck, P. Maudgal, E. De Clercq and P. Herdewijn, J. Med. Chem., 1993, 36, 538 CrossRef CAS PubMed.
- A. J. Gutierrez, T. J. Terhorst, M. D. Matteucci and B. C. Froehler, J. Am. Chem. Soc., 1994, 116, 5540 CrossRef CAS.
- R. Benhida, F. Lecubin, J. L. Fourrey, L. R. Castellanos and L. Quintero, Tetrahedron Lett., 1999, 40, 5701 CrossRef CAS.
- Y. Z. Xu, X. H. Zhang, H. C. Wu, A. Massey and P. Karran, ACS Med. Chem. Lett., 2004, 14, 995 CrossRef CAS PubMed.
- X. H. Zhang, H. Y. Yin, G. Trigiante, R. Brem, P. Karran, M. B. Pitak and Y. Z. Xu, Chem. Lett., 2015, 44, 147 CrossRef.
- M. A. Lagarkova, O. V. Iarovaia and S. V. Razin, J. Biol. Chem., 1995, 270, 20239 CrossRef CAS PubMed.
- S. Nagata, H. Nagase, K. Kawane, N. Mukae and H. Fukuyama, Cell Death Differ., 2003, 10, 108 CrossRef CAS PubMed.
- K. H. Scheit, Chem. Ber., 1968, 101, 1141 CrossRef CAS PubMed.
- P. Pozarowski and Z. Darzynkiewicz, Checkpoint Controls and Cancer: Activation and Regulation Protocols, 2004, vol. 2, pp. 301–311 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1479957. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra14356c |
| This journal is © The Royal Society of Chemistry 2016 |