Hollow Li1.2Mn0.54Ni0.13Co0.13O2 micro-spheres synthesized by a co-precipitation method as a high-performance cathode material for Li-ion batteries†
Abstract
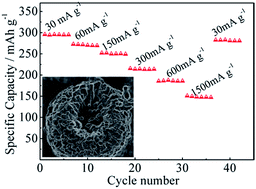
Hollow Li1.2Mn0.54Ni0.13Co0.13O2 micro-spheres were successfully synthesized by a co-precipitation method. The micro-spheres deliver an initial discharge capacity of 296 mA h g−1 and a coulombic efficiency of 83.4% at a current density of 30 mA g−1. Furthermore, the micro-spheres exhibit an excellent rate capability (150 mA h g−1 at a current density of 1500 mA g−1) and a high reversible discharge capacity of 227 mA h g−1 after 100 cycles at a current density of 60 mA g−1.

 Please wait while we load your content...
Please wait while we load your content...