Investigation on the fluorescence–(stimulus-response) properties of linear and star PVBCz-b-PDMAEMA block copolymers synthesized via ATRP†
Tengfei Mao,
Yanzi Gou*,
Hao Wang,
Ke Jian and
Jun Wang*
Science and Technology on Advanced Ceramic Fibers and Composites Laboratory, National University of Defense Technology, Changsha 410073, China. E-mail: y.gou2012@hotmail.com; wangjun_cfc@nudt.edu.cn
First published on 25th July 2016
Abstract
Linear and star PVBCz-b-PDMAEMA block copolymers with different arm numbers and PDMAEMA block lengths were successfully synthesized via ATRP using the PVBCz homopolymers with a low degree of polymerization as macro-initiators and DMAEMA as the second monomer. The properties of different PVBCz-b-PDMAEMA block copolymers were investigated including ultraviolet-visible absorption, fluorescence emission, stimulus-response and fluorescence–stimulus-response. The experimental results showed that both the arm number and PDMAEMA segment length of the block copolymers had significant effects on the properties and the special fluorescence–stimulus-responses. The block copolymers represented lower phase transition temperatures with more arm numbers and longer PDMAEMA segment length. Moreover, the phase transition temperatures also changed with respect to different pH values of the solution. The fluorescence emission intensity and emission wavelength of block copolymers varied with different ratios of THF and water, pH value and temperature of the solution. The unique function could be adjusted to meet various specific practical demands by varying the arm number and the block length, which is of great research significance. The multifunction of the block copolymers endows them with a wide application prospect, especially in the field of sensors and intelligent photoelectric materials.
Introduction
Due to their unique mechanical, viscoelastic and crystalline properties caused by the special branching architectures,1–5 star polymers have being intensively investigated with respect to the morphologies, properties and functions by varying polymeric arm types, arm numbers, and arm lengths.6,7 The well-defined branching architectures afford a more compact structure and higher segment density in comparison with linear polymeric counterparts,8,9 which are of significant importance for their applications in a variety of fields such as coatings, additives, nano-medicine,10,11 catalyst carriers,12 photonics13 and supramolecular science.14 Living anionic polymerization,15 atom transfer radical polymerization (ATRP),16 nitroxide-mediated stable free-radical polymerization (NMP),17 and reversible addition fragmentation chain transfer (RAFT) polymerization18 have been extensively applied for synthesis of well-defined star polymers through either a “core-first” method or an “arm-first” approach.19,20 The “core-first” strategy by growing arms from a multifunctional initiator core is the simplest and most efficient to synthesize star polymers of a predetermined number of arms that corresponds to the number of the initiating species of the multifunctional initiator and a well-defined arm length that is determined by feed ratio of monomer to initiator.21,22With the rapid development of living ionic polymerization and living radical polymerization (LRP) techniques in last decade, numerous work has been reported on the preparation of star block copolymers. The special properties and self-assembly performance of star block copolymers make them a terrific addition to the star homopolymers.23,24 Star block copolymers in solution can spontaneously self-assemble into various nano-ordered structures, including cylindrical, lamellar, spherical, and bicontinuous gyroid morphologies.25–28 In particular, the amphiphilic star block copolymers with hydrophobic inner blocks and hydrophilic outer blocks have attracted much attention in recent years because of their unique properties as excellent nanocarrier in pharmacological applications as well as in sensing and image enhancement.29,30
Many researchers have reported the application of using fluorescence co-monomers with aromatic units to track polymer stimuli response. Soutar and Swanson prepared copolymers by employing acenaphthylene and N-isopropyl acrylamide (NIPAM) as the monomers. And time-resolved fluorescence anisotropy was used to investigate the interaction of copolymers with sodium dodecyl sulphate31 and cononsolvency of copolymers in mixture solutions.32 Moreover, Swason also reported on detecting the formation of interpolymer complexes between poly(acrylic acid) and poly(acrylamide) by using a copolymerized napthalene derivative.33
Carbazole-containing polymers have special molecular structures with rigid fused rings and large π-conjugated systems possessing strong intra-molecular electron transfer. The special structures endow carbazole-containing polymers various distinct photoelectric functions and properties such as high charge-carrier abilities, fluorescent and photoconductive features, as well as high thermal stability.34–36 Poly[(dimethylamino)ethyl methacrylate] (PDMAEMA) is a typical “stimuli-responsive” or “smart” polymer due to the remarkable responsive behavior to the environmental stimuli such as temperature, pH and ionic strength.37,38 Therefore, the star block copolymers consisting of both carbazole-containing segments and PDMAEMA segments can exhibit amphiphilic properties. In addition, it can be expected to achieve functional coupling, which can render the copolymer potential application in sensors, intelligent photoelectric functional materials. However, to the best of our knowledge, scientific and applied research in this area has rarely been publicly reported before.
By employing (9-(4-vinylbenzyl)-9H-carbazole) (VBCz) and DMAEMA as the monomers, linear and star block copolymers with different number of arms and arm lengths were prepared in this work via ATRP through “core-first” approach. The properties of different PVBCz-b-PDMAEMA star block copolymers were investigated including ultraviolet-visible absorption, fluorescence emission, stimulus-response and fluorescence–stimulus-response. The effect of molecular architectures of the block copolymers on the fluorescent and stimuli-responsive properties was explored in detail.
Experimental section
Materials and methods
1-(Chloromethyl)-4-ethenyl-benzene was purchased from J&K, which was passed through a short column of alumina to remove inhibitors, and was finally purified by distillation under vacuum prior to use. (Dimethylamino)ethyl methacrylate (DMAEMA) (J&K, 98%) was purified through an alumina column to remove inhibitor. Copper(I) bromide (CuBr) (J&K, 99%) was purified by stirring overnight in acetic acid, followed by filtration, washing with ethanol and ether, and then drying under vacuum. Cyclohexanone (AR, Sinopharm) was dried using magnesium sulfate overnight and distilled in vacuum. Tetrahydrofuran (THF) (AR grade) was purchased from Sinopharm. Anhydrous THF was obtained by distillation under argon from sodium benzophenoneketyl. Triethylamine (TEA) (Sinopharm, AR grade) was distilled from CaH2 prior to use. Deuterated chloroform (CDCl3) + 1% v/v tetramethylsilane (TMS) (D, 99.8%) was purchased from Cambridge Isotope Laboratories. All other reagents were analytic pure and were used without further purification.All reactions were carried out using standard Schlenk techniques under an inert atmosphere of oxygen-free nitrogen, unless otherwise stated. 1H-NMR spectra were measured on Agilent 400/54 NMR instrument. The FT-IR spectra were recorded on a Perkin-Elmer 2000 spectrometer. Molecular weight and the dispersity (ĐM) were measured using a Wyatt gel permeation chromatography (GPC) equipped with a differential refractive index (DRI) detector, calibrated using PS standard samples. THF was used as the eluent at a flow rate of 1 mL min−1 operated at 25 °C. Fluorescence (FL) spectra were recorded with a Horiba JY FL-3 spectrometer. UV-vis absorption spectra were recorded with Hitachi U2810 spectrometer for solution samples. LCST (Low Critical Solution Temperature) of block copolymers was measured by monitoring the transmittance of a 550 nm light beam on a UV-Probe 3100 UV-Visible spectrophotometer (Shimadzu). The polymer concentration in water was 0.1 wt%, and the temperature was raised from 25 to 65 °C in increments of 5 °C every time (2 °C around LCST). Each sample kept stable at setting temperature for 3–5 min before recording.
Synthetic procedures
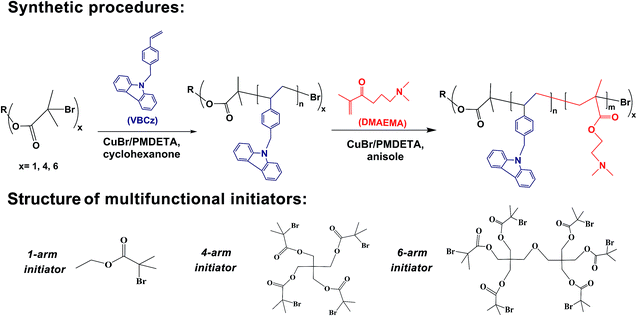
Linear and star PVBCz were directly synthesized by ATRP of VBCz using multifunctional initiators. The synthetic procedure of 4-arm PVBCz is exemplified as follows. VBCz (2.27 g, 8.00 mmol), PMEDTA (225.1 μL, 0.54 mmol), tetra-functional initiator (197.5 mg, 0.27 mmol) were charged into a dry Schlenk flask with cyclohexanone (10.0 mL) as solvent. The flask was subjected to three freeze–pump–thaw cycles to remove air completely. The solution was then transferred via cannula under nitrogen into another Schlenk flask, which was previously evacuated and filled with nitrogen, containing Cu(I)Br (37.8 mg, 0.27 mmol) and a stir bar. The reaction temperature was adjusted at 90 °C under constant stirring. At the end of polymerization, the reaction mixture was diluted with THF, and bubbled through with air for 30 min. The mixture was passed through a short neutral alumina column and subsequently washed with THF to remove the copper catalyst. The resulting polymer solution was concentrated by rotary evaporation, followed by being precipitated into methanol. The white precipitate was filtered and dried under vacuum overnight at 50 °C. The crude polymers were purified by a Soxhlet extractor with ethanol to remove the remaining VBCz. The 1- and 6-arm PVBCz were prepared using the similar procedure to the synthesis of 4-arm PVBCz with the exception that EBiB and hexa-functional initiator were used in place of tetra-functional initiator, respectively (Scheme 1). The reaction conditions were listed in Table S1 (ESI).†
Results and discussion
Preparation of the PVBCz-b-PDMAEMA block copolymers
PVBCz has strong fluorescence and specific electrochemical properties that render it particularly attractive for a variety of optoelectronic application.43,44 The star conjugated copolymers HCP-star-PDMAEMA with different PDMAEMA chain lengths synthesized from the hyperbranched conjugated carbazole polymer macro initiator via ATRP exhibited interesting thermoresponsive phase transition behaviors with adjustable LCST.45 Herein, we report the block copolymers PVBCz-b-PDMAEMA with different arm numbers and lengths completely using ATRP. Linear PVBCz-b-PDMAEMA has been reported in our preceding article,46 while the relatively long PVBCz chain led to poor solubility in water. The PVBCz with low degree of polymerization was employed as macro-initiators in this article and thus to good solubility.The synthetic routes of linear and star PVBCz homopolymers, and PVBCz-b-PDMAEMA block copolymers are shown in Scheme 1. The GPC traces of all the PVBCz homopolymers are shown in Fig. 1. Meanwhile, the molecular weight parameters of the PVBCz star polymers are listed in Table 1. The dispersity of the PVBCz is relatively narrow (1.16–1.32). The number-average degree of polymerization (DP) per arm of the star PVBCz was limited to 6 to 8 in this case to ensure good initiating activity for subsequent copolymerization with the second-monomer DMAEMA.
| Sample | Mna (×103 g mol−1) | Mwa (×103 g mol−1) | ĐMa | DPb | DParmc |
|---|---|---|---|---|---|
| a Determined by GPC.b DP, the number-average degree of polymerization of PVBCz.c DParm, the number-average degree of polymerization of PVBCz on every arm. | |||||
| 1-Arm PVBCz | 1.89 | 2.19 | 1.16 | 7 | 7 |
| 4-Arm PVBCz | 8.26 | 10.45 | 1.27 | 29 | 7 |
| 6-Arm PVBCz | 13.43 | 17.74 | 1.32 | 47 | 8 |
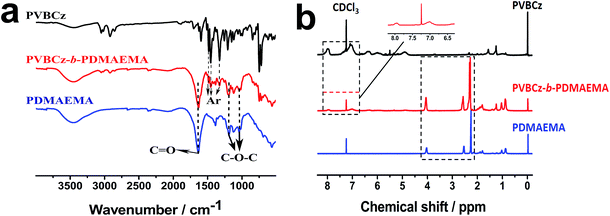
PVBCz-b-PDMAEMA block copolymers with different architectures were synthesized using corresponding PVBCz as macro-initiator, the PDMAEMA lengths of which were adjusted by varying the feed ratio of DMAEMA to macro-initiator and reaction time. Specifically, when the feed ratio was set as 100, 500 and 800, the corresponding 4-arm block copolymers were named as L4-1, L4-2 and L4-3, respectively. The FT-IR spectra of PVBCz, PDMAEMA and PVBCz-b-PDMAEMA (L4-1) are given in Fig. 2(a). The characteristic peaks at 1600, 1580, and 1450 cm−1 from aromatic groups in the chain of PVBCz can be observed in both PVBCz and PVBCz-b-PDMAEMA.47 The absorptions at 1750 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration) and 1300, 1100 cm−1 (C–O–C stretching vibration) in the spectrum of PVBCz-b-PDMAEMA indicate the presence of the PDMAEMA segment in the copolymer,47 which confirm the successful copolymerization of DMAEMA monomer.
O stretching vibration) and 1300, 1100 cm−1 (C–O–C stretching vibration) in the spectrum of PVBCz-b-PDMAEMA indicate the presence of the PDMAEMA segment in the copolymer,47 which confirm the successful copolymerization of DMAEMA monomer.
The 1H-NMR spectra of PVBCz, PDMAEMA and PVBCz-b-PDMAEMA (L4-1) in CDCl3 are shown in Fig. 2(b). The peaks at 0.80–1.05, 1.74–1.92, 2.28, 2.57 and 4.05 ppm are assigned to the methyl, methylene, dimethylamino, methylene (adjacent to the primary amino group) and oxymethylene of the PDMAEMA segment, respectively.48 Comparing with the 1H-NMR spectrum of the PVBCz, it can be identified that the weak peaks at 7.0–8.2 ppm are attributed to the carbazole aromatic protons and phenyl end group. Noting that PDMAEMA has a much longer chain than PVBCz in the copolymer L4-1, weak signals from the carbazole proton resonances are thus observed. These results are in good agreement with the FT-IR observations.
The 1H-NMR spectra and GPC traces of L4-1, L4-2 and L4-3 are shown in Fig. 3. The Mn values of the L4-1, L4-2 and L4-3 are 25200, 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, 81
500, 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 with corresponding ĐM values of 1.32, 1.36 and 1.13, respectively, as shown in Table 2. The estimated DMAEMA repeating units on every arm of the L4-1, L4-2 and L4-3 block copolymers are 27, 88 and 117, separately. Meanwhile, the block ratios of PVBCz to PDMAEMA can also be calculated according to the Mn value and 1H-NMR spectra, which are listed in Table 2. It can be found that the variation trends of the block ratio calculated by these two different methods are in good consistence, although the values are slightly different. Moreover, the block copolymers named as L1-1, L1-2 and L1-3 (1-arm), L6-1, L6-2 and L6-3 (6-arm) were obtained. Their polymerization conditions, 1H-NMR and GPC results are shown in Tables 2, S2 and Fig. S2 (ESI).† In the subsequent discussion, L1-1, L1-2 and L1-3 will be exemplified as copolymers with same arm number but different PDMAEMA segment lengths (or block ratio), while L1-1, L4-1 and L6-1 as copolymers with different arm numbers but similar PDMAEMA block lengths.
700 g mol−1 with corresponding ĐM values of 1.32, 1.36 and 1.13, respectively, as shown in Table 2. The estimated DMAEMA repeating units on every arm of the L4-1, L4-2 and L4-3 block copolymers are 27, 88 and 117, separately. Meanwhile, the block ratios of PVBCz to PDMAEMA can also be calculated according to the Mn value and 1H-NMR spectra, which are listed in Table 2. It can be found that the variation trends of the block ratio calculated by these two different methods are in good consistence, although the values are slightly different. Moreover, the block copolymers named as L1-1, L1-2 and L1-3 (1-arm), L6-1, L6-2 and L6-3 (6-arm) were obtained. Their polymerization conditions, 1H-NMR and GPC results are shown in Tables 2, S2 and Fig. S2 (ESI).† In the subsequent discussion, L1-1, L1-2 and L1-3 will be exemplified as copolymers with same arm number but different PDMAEMA segment lengths (or block ratio), while L1-1, L4-1 and L6-1 as copolymers with different arm numbers but similar PDMAEMA block lengths.
 | ||
| Fig. 3 1H-NMR spectra (a) and GPC traces (b) of the 4-arm PVBCz-b-PDMAEMA with different PDMAEMA segment length. | ||
| Samplea | Feed ratiob | Reaction time (h) | Mnc (×103 g mol−1) | Mwc (×103 g mol−1) | ĐMc | DParmd | Ratioarm,GPCe | Ratioarm,NMRf |
|---|---|---|---|---|---|---|---|---|
| a The samples are named as L (number of arm)-(serial number in terms of the length of each arm).b Feed ratio is the ratio of VBCz to the initiator.c Determined by GPC.d DP, the number-average degree of polymerization of PVBCz. DParm, the number-average degree of polymerization of PVBCz on every arm.e Ratioarm,GPC, the block ratios of PVBCz to PDMAEMA calculated according to the GPC results.f Ratioarm,NMR, the block ratios calculated according to the 1H-NMR spectra. | ||||||||
| L1-1 | 100 | 2 | 7.08 | 10.05 | 1.42 | 7 + 33 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7 4.7 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
| L1-2 | 500 | 5 | 17.14 | 20.54 | 1.20 | 7 + 97 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.9 13.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5 12.5 |
| L1-3 | 800 | 7 | 23.11 | 26.57 | 1.15 | 7 + 135 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.3 19.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.0 17.0 |
| L4-1 | 100 | 2 | 25.24 | 33.32 | 1.32 | 7 + 27 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.9 3.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2 4.2 |
| L4-2 | 500 | 5 | 63.47 | 86.38 | 1.36 | 7 + 88 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.6 12.6 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.7 13.7 |
| L4-3 | 800 | 7 | 81.71 | 92.33 | 1.13 | 7 + 117 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.7 16.7 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20.1 20.1 |
| L6-1 | 100 | 2 | 41.73 | 58.01 | 1.39 | 8 + 30 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.8 3.8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4 4.4 |
UV-vis absorption properties of the block copolymers
As shown in Fig. 4, the block copolymers exhibit maximum UV-vis absorption at about 292 nm in dilute THF solution, which can be attributed to the π–π* transition of the aromatic chromophores including carbazole units and benzene rings.44 Additionally, the spectra show two shoulder peaks at around 330 nm and 345 nm, the characteristic absorption peaks of carbazole molecules.49 By copolymerization of DMAEMA, the polymers show little red-shift in the UV-vis absorption spectra, which indicates the weak influence of PDMAEMA arms on the backbone conformation of PVBCz segments in THF solution. As shown in Fig. 4(a), the maximum absorption peak of L1-1 is at 293 nm, while that of L6-1 is at 297 nm. The maximum absorption peaks of L1-1, L4-1 and L6-1 exhibit little red-shift as the arm numbers increasing, which could be attributed to the stronger interaction of neighboring chromophores in the polymer chains.50 Fig. 4(b) shows that the maximum absorption peaks of 1-arm block copolymers have red-shift from 293 nm to 298 nm as the PDMAEMA chain lengthening. The results indicate that both the arm numbers increasing and PDMAEMA block lengthening of the block copolymers can lead to closer molecular aggregation state and stronger interaction of chromophores in THF solution. | ||
| Fig. 4 UV-vis absorption spectra of PVBCz-b-PDMAEMA in THF solution (0.1 wt%). ((a) L1-1, L4-1, L6-1; (b) L1-1, L1-2, L1-3). | ||
Fluorescent properties of the block copolymers
The fluorescent (FL) properties of the PVBCz-b-PDMAEMA block copolymers were investigated through excitation at 292 nm. The results are shown in Fig. 5. The FL emission peaks of L1-1, L4-1 and L6-1 are almost the same in the Fig. 5(a), indicating that the arm number rarely affects the FL properties of the block copolymers in THF solution. However, the block copolymers with various PDMAEMA block length exhibit distinct different FL emission (Fig. 5(b)). Specifically, the fluorescent emission of 1-arm PVBCz exhibit a slight red-shift compared with that of VBCz, which is a result of the interaction of neighboring chromophores in the polymer chains. And the FL spectrum of the copolymer L1-1 is very similar to 1-arm PVBCz, while the FL spectra of L1-2 and L1-3 are discrepant. The intrinsic FL emission peak at 351 nm vanishes and two peaks appear at relatively longer wavelength (about 405 nm and 430 nm). The red-shift of FL emission may be due to π–π stacking interactions of carbazole units of the star block copolymers.50 Namely, the longer PDMAEMA block can promote the aggregation and interaction of carbazole units in THF solution. | ||
| Fig. 5 Fluorescence spectra of PVBCz-b-PDMAEMA in THF solution (0.1 wt%). The excitation wavelength was 292 nm ((a) L1-1, L4-1, L6-1; (b) L1-1, L1-2, L1-3). | ||
Stimulus-responsive properties of the block copolymers
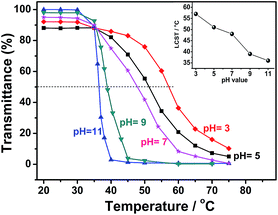
PDMAEMA homopolymer is a classical thermoresponsive polymer.37 As long as the temperature exceeds the lower critical solution temperature (LCST), which depends on the molecular weight and structure of the polymer, the polymer chains begin to collapse from coil to globule in aqueous solution.51,52 The LCST values were determined using the variable-temperature UV-vis spectrometry as the temperature at which the transmittance dropping to 50% of the original value.53 The PVBCz-b-PDMAEMA block copolymer containing PDMAEMA segments should also display the thermosensitive behavior. Moreover, as PDMAEMA is a weak polybase,38 pH value of the solution will play a marked effect on the thermoresponsive behavior of the block copolymer.Fluorescence–(stimulus-response) properties of the block copolymers
The previous sections described the optical and stimulus-responsive properties coming from PVBCz and PDMAEMA blocks, respectively. Herein, the coupling of these two properties, so-called “fluorescence–(stimulus-response) properties”, will also be investigated in detail, including the fluorescence–(solvent-response), fluorescence–(pH-response) and fluorescence–(temperature-response) properties. | ||
| Fig. 9 Fluorescence spectra and values of I/Imin of L1-1, L1-2 and L1-3 in water solution (0.1 wt%) with changing temperature (25–75 °C). The excitation wavelength was 292 nm. | ||
 | ||
| Fig. 10 Fluorescence spectra and values of I/Imin of L1-1, L4-1 and L6-1 in water solution (0.1 wt%) with changing temperature (25–75 °C). The excitation wavelength was 292 nm. | ||
Additionally, according to Fig. 9 and 10, it can be inferred that both more arm numbers and longer PDMAEMA block lead to shaper change of FL intensity as the solution temperature rising. This research is of great practical significance for applying the block copolymers to intelligent photoelectric devices and multi-function sensors, the detection range of which can be adjusted based on practical demands by varying arm numbers and PDMAEMA block length.
 | ||
| Fig. 11 Fluorescence spectra and values of I/Imin of L1-1 in aqueous solution (0.1 wt%) with changing pH value. The excitation wavelength was 292 nm. | ||
Fig. 12 shows the variation of FL intensity (I/Imin) of 0.1 wt% copolymer L1-1 in water with changing pH values at different temperatures. The corresponding pH value at the strongest FL intensity varies simultaneously with the temperature, which is found at basic pH when the temperature is below 35 °C. As the temperature is raised among 45 °C to 55 °C, the FL intensities at acidic and basic pH are both weak, which is relatively strong at neutral pH. However, the FL intensity is strong at acidic pH and decreases with the pH value when the temperature is set above 65 °C. As discussed before, the LCST of the L1-1 is strongly dependent on the solution pH, which decreases with increasing pH value. Therefore, higher temperature and larger pH value of the copolymer solutions play the same role in transforming the PDMAEMA blocks from hydrophilic to hydrophobic, leading to the higher emission intensity. At each temperature, there is a critical pH value of the transformation, corresponding to the maximal emission intensity. The unique fluorescence–(pH value-response) properties of copolymers indicate that they actually behave as a configurable fluorescent indicator of the pH window driven by temperature.
Mechanism of the fluorescence–(stimulus-response) properties
The mechanism of the unique fluorescence–(stimulus-response) properties of PVBCz-b-PDMAEMA block copolymers can uniformly be explained by Fig. 13. For conjugated copolymers, the fluorescence emission can be easily quenched by the π–π stacking of conjugated segments in self-assembly (so-called “aggregation-caused quenching” (ACQ)).54,55 In THF solution, both of the polymer segments are in the form of the coil. With water fraction increasing, the PVBCz chains aggregate to form solid-like core while the PDMAEMA segments form the shell. The FL intensity decreases due to the aggregation of the carbazole segments. With the temperature or pH value rising, the PDMAEMA segments also collapse from coil to globule. The PVBCz cores are well wrapped in the interior of the micelles by PDMAEMA segments. Therefore, the aggregation of PVBCz cores is prevented efficiently by the PDMAEMA shell, which is the reason why the FL intensity increased when the temperature was above the LCST. The ACQ effect of PVBCz cores can be further proved by the phenomenon presented in Fig. 14. The FL intensity decreases as the concentration of VBCz increasing from 1 × 10−5 to 3 × 10−4 mol L−1 in THF solution.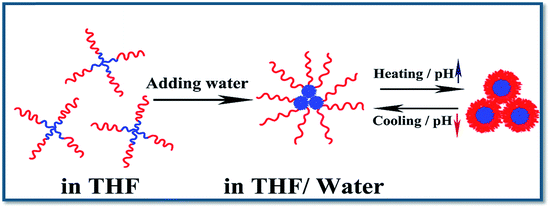 | ||
| Fig. 13 The mechanism of the unique fluorescence–(stimulus-response) properties of the PVBCz-b-PDMAEMA block copolymer. | ||
 | ||
| Fig. 14 Fluorescence spectra and values of I/Imax of VBCz at different concentration in THF. The excitation wavelength was 292 nm. | ||
Conclusions
In this work, the linear and star PVBCz homopolymers with low degree of polymerization were obtained by reducing feed ratio of monomer to initiator and shortening the reaction time. Moreover, PVBCz-b-PDMAEMA block copolymers were synthesized via ATRP using the PVBCz homopolymers as macro-initiator and DMAEMA as the second monomer. The degree of polymerization of PDMAEMA segment is available in a relatively wide range (30–140) with narrow molecular weight distribution (ĐM < 1.42).The properties of different PVBCz-b-PDMAEMA block copolymers were investigated including ultraviolet-visible absorption, fluorescence emission, stimulus-response and fluorescence–(stimulus-response) performance. Both the arm numbers and PDMAEMA block length of the block copolymer have significant effects on the performance of the resulting polymers. The ultraviolet-visible absorption peaks of different block copolymers shift comparing with those of the homopolymers. The effects on fluorescence emission are mainly reflected by the changes of relative intensity of emission peak at 350, 365 and 405 nm. The block copolymers have obvious temperature- and pH-responsive performance. The LCST of block copolymers changes with pH values. Moreover, the LCST of different block copolymers varies at the same pH value. The intensity and wavelength of fluorescence emission of block copolymers in solution change along with the solvent, pH and temperature. Both of the arm numbers and PDMAEMA block length of the block copolymer affect the special fluorescence–(stimulus-response) performance. The multifunction of the block copolymers endows them a wide application prospect, especially in the fields of sensors and intelligent photoelectric materials. Moreover, the performance could be adjusted to meet various specific practical demands by varying the arm number and the block length, which is of great research significance.
Acknowledgements
This research was sponsored by the National Natural Science Foundation of China (Grant No: 51302313) and the Fund of Science and Technology on Advanced Ceramic Fibers and Composites Laboratory (Grant No: 9140C820203140C82345, 9140C820403150C82024). The financial support from Postdoctoral Science Foundation of China (2014M552685) was appreciated as well. The authors are also thankful for the financial support from Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, and Aid Program for Innovative Group of National University of Defense Technology.Notes and references
- J. A. Syrett, D. M. Haddleton, M. R. Whittaker, T. P. Davis and C. Boyer, Chem. Commun., 2011, 47, 1449–1451 RSC.
- H. Gao and K. Matyjaszewski, Prog. Polym. Sci., 2009, 34, 317–350 CrossRef CAS.
- A. Blencowe, J. F. Tan, T. Goh and G. G. Qiao, Polymer, 2009, 50, 5–32 CrossRef CAS.
- K. Khanna, S. Varshney and A. Kakkar, Polym. Chem., 2010, 1, 1171–1185 RSC.
- D. Kuckling and A. Wycisk, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2980–2994 CrossRef CAS.
- B. M. Erwin, M. Cloitre, M. Gauthier and D. Vlassopoulos, Soft Matter, 2010, 6, 2825–2833 RSC.
- M. Sugimoto, T. Koizumi, T. Taniguchi and T. Morita, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 2226–2237 CrossRef CAS.
- A. Hult, M. Johansson and E. Malmstrom, Adv. Polym. Sci., 1999, 143, 1–34 CrossRef CAS.
- W. Burchard, Adv. Polym. Sci., 1999, 143, 113–194 CrossRef CAS.
- M. E. Fox, F. C. Szoka and J. M. Frechet, Acc. Chem. Res., 2009, 42, 1141–1151 CrossRef CAS PubMed.
- C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS PubMed.
- V. Rodionov, H. Gao, S. Scroggins, D. A. Unruh, A. J. Avestro and J. M. Frechet, J. Am. Chem. Soc., 2010, 132, 2570–2572 CrossRef CAS PubMed.
- Q. Zhao, S. J. Liu and W. Huang, Macromol. Chem. Phys., 2009, 210, 1580–1590 CrossRef CAS.
- K. Char, C. W. Frank and A. P. Gast, Macromolecules, 1989, 22, 3177–3180 CrossRef CAS.
- M. Szwarc, M. Levy and R. Milkovich, J. Am. Chem. Soc., 1956, 78, 2656–2657 CrossRef CAS.
- (a) M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721–1723 CrossRef CAS; (b) Y. Z. Gou, J. Geng, S. Richards, J. Burns, C. R. Becer and D. M. Haddleton, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2588–2597 CrossRef CAS PubMed.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661–3688 CrossRef CAS PubMed.
- (a) J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, K. Jeffery, P. T. L. Tam, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS; (b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS.
- K. Yang, H. Liang and J. Lu, J. Mater. Chem., 2011, 21, 10390–10398 RSC.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689–3745 CrossRef CAS PubMed.
- K. Matyjaszewski, D. A. Shipp, G. P. McMurtry, S. G. Gaynor and T. Pakula, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2023–2031 CrossRef CAS.
- X. C. Pang, L. Zhao, M. Akinc, J. K. Kim and Z. Q. Li, Macromolecules, 2011, 44, 3746–3752 CrossRef CAS.
- Y. H. Kim, W. T. Ford and T. H. Mourey, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4623–4634 CrossRef CAS.
- J. Rodríguez-Hernández, F. Chécot, Y. Gnanou and S. Lecommandoux, Prog. Polym. Sci., 2005, 30, 691–724 CrossRef.
- L. Zhang and A. Eisenberg, Macromolecules, 1996, 29, 8805–8815 CrossRef CAS.
- H. Shen and A. Eisenberg, Macromolecules, 2000, 33, 2561–2572 CrossRef CAS.
- H. Cui, Z. Chen, K. L. Wooley and D. J. Pochan, Macromolecules, 2006, 39, 6599–6607 CrossRef CAS.
- K. Y. Cho, J. W. Choi, S. H. Lee, S. S. Hwang and K. Y. Baek, Polym. Chem., 2013, 4, 2400–2405 RSC.
- C. Koch, A. Z. Panagiotopoulos, F. L. Verso and C. N. Likos, Soft Matter, 2015, 11, 3530–3535 RSC.
- C. K. Chee, S. Rimmer, I. Soutar and L. Swanson, Soft Matter, 2011, 7, 4705–4714 RSC.
- C. K. Chee, B. J. Hunt, S. Rimmer, I. Soutar and L. Swanson, Soft Matter, 2011, 7, 1176–1184 RSC.
- T. Swift, L. Swanson and S. Rimmer, RSC Adv., 2014, 4, 57991–57995 RSC.
- J. V. Grazulevicius, P. Strohriegl, J. Pielichowski and K. Pielichowski, Prog. Polym. Sci., 2003, 28, 1297–1353 CrossRef CAS.
- S. Krotkus, K. Kazlauskas, A. Miasojedovas, A. Gruodis, A. Tomkeviciene, J. V. Grazulevicius and S. Jursenas, J. Phys. Chem. C, 2012, 116, 7561–7572 CAS.
- F. Loiseau, S. Campagna and A. Hamaudaine, J. Am. Chem. Soc., 2005, 127, 11352–11363 CrossRef CAS PubMed.
- S. J. Chen, F. Du and Z. Q. Wu, Polym. Prepr., 1997, 38, 534–537 CAS.
- S. Cho, M. John and H. Lee, J. Polym. Sci., Part B: Polym. Phys., 1997, 35, 595–600 CrossRef CAS.
- F. Chen, G. Liu and G. Zhang, J. Phys. Chem. B, 2012, 116, 10941–10950 CrossRef CAS PubMed.
- Z. Zhang, T. Hughes, X. Hao and G. G. Qiao, Polymer, 2013, 54, 4446–4454 CrossRef CAS.
- Y. F. Tong, L. Chen, X. H. He and Y. W. Chen, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4341–4350 CrossRef CAS.
- J. L. Liu, W. W. He, L. F. Zhang, Z. B. Zhang, J. Zhu, L. Yuan, H. Chen, Z. P. Cheng and X. L. Zhu, Langmuir, 2011, 27, 12684–12692 CrossRef CAS PubMed.
- X. Zhu, N. C. Zhou, Z. B. Zhang, B. Sun and X. L. Zhu, Angew. Chem., Int. Ed., 2011, 50, 6615–6618 CrossRef CAS PubMed.
- Z. Li, P. Li and J. Huang, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4361–4371 CrossRef CAS.
- F. Qiu, D. Wang, R. Wang, X. Huan, G. Tong, Q. Zhu, D. Yan and X. Zhu, Biomacromolecules, 2013, 14, 1678–1686 CrossRef CAS PubMed.
- T. F. Mao, Y. Z. Gou and J. Wang, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 87, 1–8 Search PubMed.
- Z. Cheng, X. Zhu and K. G. Neoh, Macromolecules, 2005, 38, 7187–7192 CrossRef CAS.
- X. Zhang, J. Xia and K. Matyjaszewski, Macromolecules, 1998, 31, 5167–5169 CrossRef CAS PubMed.
- J. P. Chen and A. Natansohn, Macromolecules, 1999, 32, 3171–3177 CrossRef CAS.
- W. Zhang, Y. Yan, N. Zhou, Z. Cheng, J. Zhu, C. Xia and X. L. Zhu, Eur. Polym. J., 2008, 44, 3300–3305 CrossRef CAS.
- D. Fournier, R. Hoogenboom, H. M. L. Thijs, R. M. Paulus and U. S. Schubert, Macromolecules, 2007, 40, 915–920 CrossRef CAS.
- F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Müller, J. Am. Chem. Soc., 2007, 129, 14538–14539 CrossRef CAS PubMed.
- P. Y. Gu, Y. H. Zhang, D. Y. Chen, C. J. Lu, F. Zhou, Q. F. Xu and J. M. Lu, RSC Adv., 2015, 5, 8167–8174 RSC.
- S. T. Lin, Y. C. Tung and W. C. Chen, J. Mater. Chem., 2008, 18, 3985–3992 RSC.
- S. T. Lin, K. Fuchise and R. Sakai, Soft Matter, 2009, 5, 3761–3770 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Figures and tables showing additional 1H-NMR and UV-vis spectra, reaction conditions, and molecular weight parameters of the PVBCz macro initiators and PVBCz-b-PDMAEMA block copolymers. See DOI: 10.1039/c6ra14316d |
| This journal is © The Royal Society of Chemistry 2016 |