A step ahead towards the green synthesis of monodisperse gold nanoparticles: the use of crude glycerol as a greener and low-cost reducing agent†
Rashida Parveen and
Germano Tremiliosi-Filho*
Institute of Chemistry of São Carlos, University of São Paulo, P. O. Box 780, CEP 13560-970, São Carlos, SP, Brazil. E-mail: germano@iqsc.usp.br; Tel: +55-16-3373-9951
First published on 28th September 2016
Abstract
Owing to their widespread application, the preparation of monodisperse gold nanoparticle (AuNPs) by green methods and using low-cost and environment friendly reagents is of great importance. In this study, the formation of nearly monodisperse spherical AuNPs of around 8 nm has been achieved, for the first time, using the as-received crude glycerol (CG) from the biodiesel plant. As a proof of concept, two different crude glycerol samples with different glycerol contents (65% and 73%) and different impurities levels (low and high) and types (organic and inorganic) were employed to prepare AuNPs. No special chemical or physical treatment of CG except simple filtration was carried out. The effect of possible impurities in CG as well as synthesis parameters (pH, glycerol concentration, and stabilizing agent concentration) on the shape and size distribution of AuNPs was studied. The shape and particle size distribution of AuNPs was found to be greatly affected by the concentration of stabilizing agent polyvinylpyrrolidone (PVP) and polydiallyldimethylammonium chloride (PDAC) while the number of particles formed is strongly dependent on pH of the reaction media. Uniformly distributed AuNPs of around 7 ± 1.5 nm were produced under a wide range of glycerol concentration (0.1–0.4 mol L−1) and OH− concentration (0.1–0.4 mol L−1) using 1.0% stabilizing agents concentration. Both PVP and PDAC, besides acting as stabilizing agents, also help in reduction of metal salts in basic media. For comparison, AuNPs were also prepared using commercial glycerol (purity ≥ 99.5%) under identical experimental conditions. AuNPs with similar size and shape were obtained in both cases (commercial pure glycerol as well as CG of varying glycerol content and impurities) indicating that commercial glycerol can be replaced with CG in the AuNPs synthesis and the organic and inorganic impurities do not significantly affect the particle size distribution of prepared AuNPs. This study opens up new possibilities for the environment-friendly preparation of metallic nanoparticles using the low-cost, non-toxic and biodegradable CG as a reducing agent.
1. Introduction
The synthesis of gold nanoparticles (AuNPs) especially with a control of size and shape has attracted great attention due to their ultrafine dimensions and unique size- and shape-dependent properties1–4 and low toxicity.5,6 These unique properties have led to the widespread application of size- and shape-controlled AuNPs in catalysis,7–12 sensing,13,14 photothermal therapy,15–17 drug delivery,4,18 bioimaging and other biomedical applications.18–20 Thus controlling the NPs shape and size allows for tuning of such size- and shape-dependent properties.Due to these unique optical and catalytic properties, various methods have been developed for the preparation of AuNPs in various forms and shapes. These methods vary considerably with respect to the reproducibility, yield, purity and shape-control of AuNPs as well as environmental safety. Such different methodologies include template,21 photochemical,22–24 electrochemical,25,26 microwave irradiation27 and wet chemical methods.28–30 The common wet chemical reduction methods for the preparation of AuNPs generally employ a reducing agent such as sodium borohydride (NaBH4) or hydrazine to reduce the metal salts to metal NPs. In addition to their carcinogenic nature, both these reducing agents are expensive, difficult to handle and cannot be stored for long time because they decompose with time. Thus, the development of simpler methods employing low-cost, safe and easily handled reagents is of greater importance. Such attempts include reduction of metal salts to metallic nanoparticles using plant extracts,31,32 microorganisms33,34 and environment friendly reactants.28–30,35,36
Though the use of plants' extracts is a good alternative, the extraction procedures are time and energy consuming and may involve the use of organic solvents. Further, the chemical composition of plants' extracts strongly depends on the plant habitat and this leads to less reproducibility of the synthesis procedures based on plants' extracts. The employment of microorganism for nanoparticle synthesis also requires strict control of pH and temperature as the growth of microorganisms strongly depends on these conditions.
Our research group has recently reported the preparation of Au and Ag nanoparticles using high purity commercial glycerol in alkaline media as a low-cost and greener reducing agent.28,35–39 The use of glycerol as a low-cost and greener reducing agent is an attractive option to prepare AuNPs. Genc et al. reported the preparation of AuNPs using liposomes as nanoreactors and glycerol, incorporated within the nanoreactors, as reducing agent to reduce HAuCl4 inside the liposomes.30 Small AuNPs of 2–8 nm were prepared by this liposomes-based method employing 6-mercapto-1-hexanol and PVP as capping agents. The formation of liposomes (nanoreactors) by the curvature-tuned preparation,40 which requires a strict control of lipid composition and temperature and a rapid pH jump from pH 7.4 to pH 11 and back to pH 7.4, however, makes the procedure difficult and time consuming.30 Nalawade et al. also reported the preparation of AuNPs in the 8–50 nm size range using neat glycerol or glycerol–PVP mixture.28 Similarly, Grace and Pandian employed glycerol as a reducing agent to prepare PVP-protected AuNPs using microwave and reflux methods to boil the reaction mixture to glycerol boiling point (290 °C).29 Spherical AuNPs of around 7 nm were formed under reflux conditions at 290 °C while triangular prismatic gold nanoparticles were formed at the same temperature under microwave mode of heating.29
From this brief literature review, it becomes clear that glycerol has the potential to be employed as low-cost and greener reducing agent. However, the reported studies either employed commercial high purity glycerol (purity ≥ 99.5%)28,35,37,38,41 or require costly instruments,29 expensive reagents30 or complicated procedures.30 In the present study, we employed CG obtained directly from the transesterification reaction to prepare AuNPs. Furthermore, no purification step except simple filtration was used to purify the CG.
The motivation behind the present work was (i) to replace the less stable and hazardous reducing agents such as sodium borohydride and hydrazine with the environmentally benign, chemically stable, biodegradable and abundantly available glycerol, especially in its crude form, (ii) to explore new possibilities for the preparation of metallic nanoparticles using CG as a reducing agent, (iii) to compare the efficiency of commercial (high purity) and CG in the synthesis of AuNPs and (iv) to benefit from the sustainable nature of glycerol and employing it as a low-cost and abundantly available reducing agent.
CG is the main by-product of transesterification reaction in biodiesel industry42,43 and saponification reaction in soap industry.44 This by-product finds many industrial applications in the production of cosmetics, resins, medicines, foods, fabrics and value-added chemicals45 etc. However, for such applications, the glycerol must be purified and this purification process is a major technological and economic concern in biodiesel industry. Furthermore, as biodiesel is produced in large scale, excess of CG as by-product (2 billion lbs in 2009)43 is produced which makes glycerol a very economic reagent for applications in chemical processes and reactions. Though the quantity of glycerol used in our synthesis procedure is very small, it can be regarded as a sustainable reducing agent/solvent.42,43,45
In this study, we are going to demonstrate that CG coming from the trans-esterification reaction42 in biodiesel industries can be successfully employed as a reducing agent to prepare AuNPs of less than 10 nm with good size homogeneity. Two different CG sample with different glycerol content (73% and 65%) and different impurities level (high and low) were employed to prepare AuNPs and AgNPs. Furthermore, the effect of two different surfactants (PVP or PDAC), reaction pH and CG concentration on the shape and size distribution and stability of AuNPs is discussed. This one-pot simple procedure employing the low-cost CG falls within the principles of green chemistry and offers the possibility of synthesis of Au and other metallic nanoparticles in an environment friendly way.
2. Experimental
2.1. Chemical reagents
Polyvinylpyrrolidone, PVP, (average molecular weight 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), polydiallyldimethylammonium chloride, PDAC, (20 wt% in H2O, molecular weight 100
000 g mol−1), polydiallyldimethylammonium chloride, PDAC, (20 wt% in H2O, molecular weight 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–200
000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and HAuCl4 were purchased from Sigma Aldrich. Commercial glycerol (∼99.5%) and NaOH were purchased from Panreac. All these chemicals were of analytical grade and used without further purification. CG (locally known as Glicerina Loira) samples with a brownish yellow colour and derived from a mixture of plant oils and animal fats were obtained from BioBrotas Oleoquímica Industry (Brotas, SP, Brazil). The CG-73 sample was first vacuum filtered through a 7 μm filter paper and then through a 0.2 μm syringe filter to remove the particulate matter. The CG-65 was used as such, without any pre-treatment. All aqueous solutions used in the synthesis were prepared with high purity water (18.2 MΩ cm) purified by Millipore Milli-Q Plus system. The glassware and plastic tubes used in the experiments were cleaned first with permanganate solution, then with piranha solution (3 mL H2SO4
000 g mol−1) and HAuCl4 were purchased from Sigma Aldrich. Commercial glycerol (∼99.5%) and NaOH were purchased from Panreac. All these chemicals were of analytical grade and used without further purification. CG (locally known as Glicerina Loira) samples with a brownish yellow colour and derived from a mixture of plant oils and animal fats were obtained from BioBrotas Oleoquímica Industry (Brotas, SP, Brazil). The CG-73 sample was first vacuum filtered through a 7 μm filter paper and then through a 0.2 μm syringe filter to remove the particulate matter. The CG-65 was used as such, without any pre-treatment. All aqueous solutions used in the synthesis were prepared with high purity water (18.2 MΩ cm) purified by Millipore Milli-Q Plus system. The glassware and plastic tubes used in the experiments were cleaned first with permanganate solution, then with piranha solution (3 mL H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL H2O2) and finally washed with copious amount of Milli-Q water.
1 mL H2O2) and finally washed with copious amount of Milli-Q water.
2.2. Characterization of CG
The glycerol content of the CG samples was analyzed using high-performance liquid chromatography (HPLC, Shimadzu) equipped with a pump (LC-10ADVP), auto sampler (SIL-20A HT), column oven (CTO-20A) at 43 °C, refractive index detector (RID-10A), system controller (SCL-10AVP) and an Aminex HPX-87H column (300 × 7.8 mm, BioRad). Aqueous solution of H2SO4 (5 mmol L−1) was used as the mobile phase. For HPLC analysis, 35.2 mg of filtered CG sample was diluted to 25 mL in a volumetric flask and injected into HPLC. The glycerol content (X) of the sample was obtained by comparing the area of glycerol peak at retention time of 16.1 min in the chromatogram with that of a series of standards obtained under the same conditions using a calibration curve. The glycerol content (X) was calculated using the formula: X = Y − C/S, where Y is the peak area of the sample, S is the slope and C is the intercept of calibration curve. The glycerol content of CG-73 thus obtained from HPLC analysis was 73% (9.8 mol L−1).
| Sample | % glycerol | % water | Density (g mL−1) | Colour | pH | Organica and other impurities |
|---|---|---|---|---|---|---|
| a Relative percentage of organic impurities calculated from GC-MS analysis. | ||||||
| Commercial glycerol (Panreac) | 99.0–99.5 | 0.39 ± 0.1 | 1.26 | Transparent | ∼5 (100 g L−1) | cis-Vaccenic acid (0.48%) |
| CG-73 | 72.9 | 17.8 ± 1.4 | 1.25 | Yellow | 10 ± 1 | Traces of fatty acid and soap, 7% salt |
| CG-65 | 65 | 3.49 ± 0.1 | 1.03 | Yellow | 10 ± 1 | Methyl dodecanoate (4%), dodecanoic acid (1.8%), n-hexadecanoic acid (1.1%), 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (3.5%), methyl (E)-octadec-11-enoate (13.6%), cis-vaccenic acid (8.4%), 9-octadecenoic acid, 1,2,3-propanetriyl ester (2.3%) |
2.3. Preparation of AuNPs by one-pot method
The details of the experimental conditions used in the synthesis of the AuNPs by the one-pot method using CG are reported in Table 2. Typically, different volumes (0.5–2.0 mL) of CG were diluted to 50 mL with NaOH solution in a polypropylene plastic tube to obtain basic glycerol solution of different concentration (0.1–0.4 mol L−1). The pH of glycerol solution was varied by using NaOH solution of different concentrations (0.1–0.4 mol L−1). Then 2 mL of this basic glycerol solution was added to a mixture consisting of 5 mL stabilizing agent (PVP or PDAC, both 1%) and 5.0 mL of HAuCl4 (7.5 × 10−4 mol L−1) in a polypropylene tube, which resulted in the formation of AuNPs as indicated by the appearance of pinkish red colour. After the formation of AuNPs was complete, the samples were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes and 13
000 rpm for 10 minutes and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 minutes to remove excess surfactant.
000 rpm for 30 minutes to remove excess surfactant.
| Parameter | Glycerol (mol L−1) | NaOHa (mol L−1) | PVP% (wt/vol) | PDAC% (wt/vol) |
|---|---|---|---|---|
| a NaOH concentration used in the preparation of glycerol solution to adjust the pH. | ||||
| Effect of PVP | 0.2 | 0.3 | 0.03, 0.05, 1, 1.3 | — |
| Effect of glycerol | 0.1,0.2, 0.3, 0.4 | 0.3 | 1 | — |
| Effect of NaOH (pH) | 0.2 | 0.1, 0.2, 0.3, 0.4 | 1 | — |
| Effect of PDAC | 0.2 | 0.3 | — | 0.03, 0.05, 1, 1.3 |
| Effect of NaOH (pH) | 0.2 | 0.1, 0.2, 0.3, 0.4 | — | 1 |
2.4. Characterization of the AuNPs
The formation of AuNPs was monitored by taking electronic absorption spectra using a UV-Vis-NIR spectrophotometer (Shimadzu). Transmission electron microscopy (TEM) images were obtained using an FEI TECNAI (G2F20) transmission electron microscope operated at an accelerating voltage of 200 kV and equipped with an energy-dispersive X-ray (EDX) detector. For the TEM study, samples were prepared by drop-casting 10 μL of the AuNPs suspension onto a carbon-coated copper grid (CFC-200Cu, Electron Microscopy Sciences, USA). Field emission gun-scanning electron microscopy (FEG-SEM) images were obtained using Inspect F-50 microscope (FEI, Nederland) at an electron beam accelerating voltage of 15 kV. The samples were deposited from dilute aqueous suspension as a thin layer on single-crystal silicon slabs and allowed to dry under ambient conditions. The mean diameter of the AuNPs was calculated by measuring the diameter of at least 200 AuNPs from the TEM and FEG-SEM images. The particle size distribution (by number) was also measured by dynamic light scattering (DLS) technique using Zetatrac DLS system (Microtrac, USA) equipped with a diode laser emitting at 780 nm. The run time and number of runs used for each sample measurement were 60 s and 3 runs respectively. X-ray diffraction (XRD) measurements of the AuNPs supported on vulcan carbon were carried out using a Rigaku diffractometer. In order to characterize glycerol and the possible impurities present in it, FTIR spectra in the range 400–4000 cm−1 at a spectral resolution of 4 cm−1 were acquired using an MB-102 (Bomem, Canada) spectrophotometer. The 13C NMR spectra of the glycerol samples were acquired with an Agilent Technologies 400/54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 MHz NMR apparatus at 25 °C. The GC-MS analysis of CG was performed using GCMS-QP2010 Ultra (Shimadzu) Gas Chromatograph Mass Spectrometer equipped with MS-5 30 m × 0.2 mm column. The temperature programming was done by keeping the temperature at 80 °C for two min and then increased from 80 °C to 300 °C at a heating rate of 10 °C min−1.
400 MHz NMR apparatus at 25 °C. The GC-MS analysis of CG was performed using GCMS-QP2010 Ultra (Shimadzu) Gas Chromatograph Mass Spectrometer equipped with MS-5 30 m × 0.2 mm column. The temperature programming was done by keeping the temperature at 80 °C for two min and then increased from 80 °C to 300 °C at a heating rate of 10 °C min−1.
3. Results and discussion
The AuNPs were prepared using CG in alkaline media as a reducing agent and two different polyelectrolytes (PVP or PDAC) as particle stabilizing agents. The effect of impurities in CG, concentration of glycerol and capping agent and pH on particle size, size distribution and shape of AuNPs has been studied. Importantly, to check if the CG obtained directly from transesterification reaction has the same (or different) efficiency as a reducing agent and results in the formation of similar (or different) AuNPs, AuNPs were also synthesized using commercial glycerol (99.5%, Panreac) under the same experimental conditions as the CG. The results obtained showed that CG can be employed to obtain AuNPs of similar crystallite size and crystallinity as that of pure glycerol. The details of the synthesis of AuNPs using CG are presented below.3.1. Preparation of AuNPs using CG and PVP
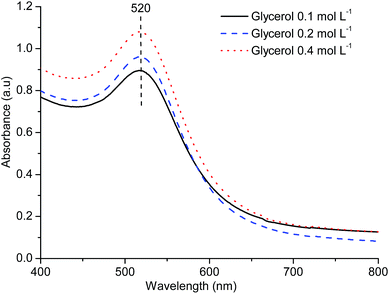
To further verify the effect of glycerol concentration on particle size of AuNPs, DLS analysis of samples prepared using different concentrations of glycerol was done (Fig. 2). Particle sizes measured using DLS confirmed that all samples show an average particle size of around 9 nm and that different initial glycerol concentrations have no considerable effect on the particle size distribution, in agreement with the results from electronic absorption spectroscopy.
TEM analysis was also performed to measure particle size of AuNPs prepared with different initial glycerol concentrations. The particle sizes of various samples measured using electron microscopy (Fig. S7†) agree closely with the hydrodynamic diameters measured by DLS analysis (Fig. 2). The HRTEM images of the AuNPs prepared using CG-73 showed the polycrystalline nature of the spherical particles with crystal defects (Fig. S8†) as also observed previously in case of AuNPs prepared with pure glycerol.37
For the sake of comparison, AuNPs were also prepared using commercial glycerol of high purity (99.5%, Panreac) (Fig. 3a) and CG with varying level of organic and inorganic impurities (Fig. 3b and c), keeping other experimental conditions constant (Fig. 3). From a comparison of TEM images of AuNPs prepared with commercial glycerol, CG-73 and CG-65 (Fig. 3), it is evident that the spherical AuNPs with almost similar size and distribution are obtained in case of both commercial (pure) and CG. These results indicate that CG obtained directly from transesterification process can be efficiently used to synthesize metallic nanoparticle with good size distribution and the impurities present therein do not affect the particle size of AuNPs. This green approach of utilizing CG obtained as by-product from biodiesel industry assures sustainable use of glycerol as reducing agent and solvent.
The X-ray diffractograms of the carbon-supported AuNPs prepared using both CG and commercial glycerol are shown in Fig. 4. In both cases, the XRD patterns revealed clear diffraction peaks with similar shape and relative intensity ratio at 2-theta values of 38.2°, 44.3°, 64.6°, 77.6° and 81.7° which correspond to the (111), (200), (220), (311) and (320) planes of the face-centered cubic (fcc) structure of gold (pdf # 65-2870). The average crystallite size of AuNPs estimated using Scherrer's equation was found to be 13 ± 3 nm in case of both CG-73 and commercial glycerol. This confirms that CG can be employed to obtain AuNPs of similar crystallite size and crystallinity as that of commercial glycerol of high purity and no special purification steps except simple conventional filtration through a 0.2 μm filter are required to prepare AuNPs.
Since PVP is a neutral polymer, the stabilization is expected to occur through steric means.47,48 Zhou et al. have shown that PVP actually stabilizes the AuNPs by coordination of the carbonyl oxygen of PVP with the surface of Au, as indicated by an increase in binding energy of O 1s XPS peak of carbonyl group and a slight decrease in binding energy of Au 4f XPS peak.47 Wang et al. have proposed that PVP stabilizes the AgNPs by forming a protecting layer around AgNPs as a result of coordination of silver with N atom of PVP49 for nanoparticles less than 50 nm and for bigger particles, silver can form coordination with oxygen atom of PVP as well.
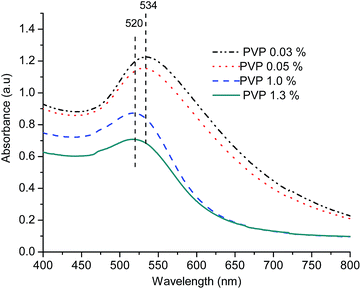
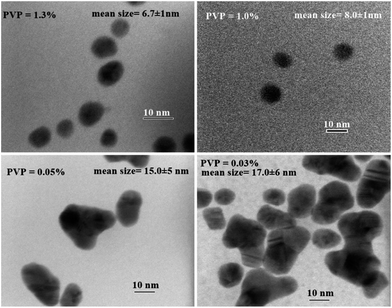
The TEM images of AuNPs prepared using different amounts of PVP clearly show the effect of PVP concentration on particle shape and size distribution. Fig. 6 shows that at higher PVP concentration (≥1%), spherical nanoparticles with uniform distribution (8 ± 1 nm) are formed, but as we decrease the PVP concentration to 0.05% or lower, bigger AuNPs (around 16 ± 6 nm) are formed due to agglomeration of the nanoparticles.
The hydrodynamic diameter of the AuNPs prepared using different PVP concentrations was also measured using DLS (Fig. S9†). The particle size obtained from DLS analysis is 6 ± 1 nm and 9 ± 1 nm for AuNPs prepared using 1.3% and 1% PVP concentration, respectively, in agreement with the results from TEM analysis.
Summarizing the results of electronic spectroscopy, TEM analysis and DLS, it is observed that the PVP concentration plays important role in the stabilization of AuNPs and hence the particle size.48 The presence of large sized aggregated particles at lower PVP also indicates that glycerol itself or the trace impurities present in it play no significant role in particle stabilization under the conditions of the experiment. The final concentration of PVP may be kept at least 0.4% or above in the reaction mixture containing AuCl4− and glycerol in order to obtain AuNPs with sufficient stability and uniform size distribution.
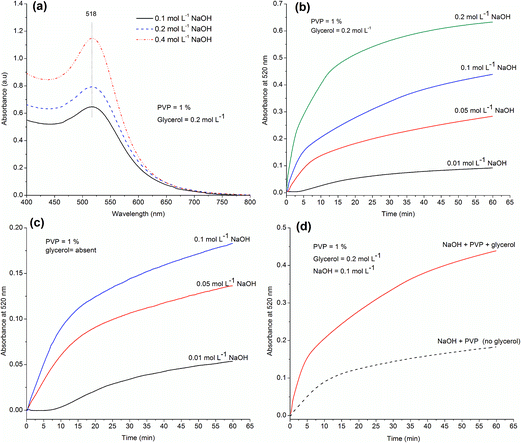
In alkaline media, both glycerol39,41 and PVP47 may act as reducing agents. Since the alkoxide species (–CO−) formed from glycerol upon deprotonation in alkaline media is the principal active reducing agent,39 its formation may be favored at higher pH which results in efficient reduction of Au3+ ions and hence nanoparticles formation, as indicated by increased absorption at higher pH (Fig. 7a). This inference is supported by the fact that the rate of formation of AuNPs is enhanced at higher pH in the reaction mixture containing glycerol (Fig. 7b). However, besides stabilizing the AuNPs, the role of PVP as reducing agent50 in alkaline media cannot be ignored; AuNPs are formed even in the absence of glycerol in the reaction mixture (Fig. 7c). NaOH may help in the abstraction of H atoms from the tertiary carbon of PVP or cause polymer degradation, producing organic radicals that may in turn take part in reduction of Au3+ ions.46,47 Furthermore, the NaOH-mediated reduction of Au3+ by PVP results in the formation of H+ which must be neutralized by OH− ions present in the reaction mixture.47 This may be one of the possible reasons for the decrease in pH as the reaction proceeds and the increase in reaction rate at higher pH. Fig. 7c shows that the reducing ability of PVP is also pH-dependent. For instance, the formation of AuNPs only starts after 7 minutes of mixing the reagents when the concentration of NaOH is low (0.01 mol L−1) but starts immediately after mixing the reagents when the NaOH concentration is higher. The slope of the growth curves is different at different pH, becoming steeper at higher pH (Fig. 7c).
Though PVP acts as reducing agents in alkaline media,47 the reduction rate is faster when glycerol is present and glycerol acts as the principal reducing agent39 (Fig. 7d). Gomes et al. have proposed that alkoxide formed from glycerol in alkaline medium is the actual and universal reducing agent for silver and gold ions and their inference is based on the fact that AuNPs could be formed using potassium tert-butoxide as the reducing agent in the absence of any hydroxide ions in the media.39 However, it is important to mention that no AuNPs were formed in the presence of only NaOH in the reaction mixture.
TEM analysis, in fact, affirmed the inferences derived from UV-Vis spectroscopy showing that all samples have an average particle size of around 7 ± 1 nm (Fig. 8). Also, the hydrodynamic diameter for the different samples prepared at different pH is around 7 ± 2 nm as measured by DLS (Fig. S10†). The little difference in size between TEM and DLS is probably related to the fact that the DLS measures the hydrodynamic diameter of a large number of particles while TEM analysis is based on measurement of particle diameter of a limited number of nanoparticles.
3.2. Preparation of AuNPs using crude glycerol (CG) and PDAC
Fig. 9a shows the electronic absorption spectra of AuNPs prepared using different concentrations of the stabilizing polyelectrolyte, PDAC, at fixed OH− concentration. The SPR absorption band lies at around 516 nm for PDAC concentration of 1% or above indicating the formation of spherical AuNPs.46 Also, there is no significant absorption above 600 nm which suggests the absence of any large aggregated particles and the formation of small particles with narrow size distribution. The λmax, however, is red-shifted and the absorbance above 600 nm becomes significant at concentration of PDAC less than 1%. This suggests the formation of comparatively bigger and longer AuNPs which show polydispersity in sizes due to insufficient capping of the growing AuNPs by PDAC at lower concentration (<1%). Again, the presence of bigger particles at lower concentration of PDAC suggests that CG itself or the possible impurities present in it have no role in the stabilization of AuNPs under the experimental conditions and a stabilizing agent must be added to obtain small monodisperse particles.As in case of PVP, the pH of the solution has little effect on the size distribution of the PDAC-stabilized AuNPs (Fig. 9b and S11†) but significantly affect the number of particles and the rate of their formation. The lowest dashed curve in Fig. 9b is the spectrum of AuNPs prepared in the absence of glycerol in the reaction mixture. The presence of a low intensity band centered at 530 nm in this spectrum shows that PDAC in alkaline media can also reduce Au3+ ions into metallic AuNPs.51
Analysis of the particle sizes by TEM (Fig. 10) and DLS (Fig. S12†), in fact, confirmed the results of electronic spectroscopy showing that AuNPs of around 8 ± 2 nm with a narrow size distribution are formed at PDAC concentration higher than 1%, while polydispersed large size particles of 14 nm and 20 nm are formed at PDAC concentration of 0.05% and 0.03%, respectively.
 | ||
| Fig. 10 TEM images of the PDAC-stabilized AuNPs obtained at different PDAC concentration (0.03%–1.3%) while keeping other parameters constant. | ||
As in case of PVP, the reducing strength of PDAC is also dependent on pH of the solution, the reaction being faster at higher OH− concentration (Fig. 11a and S13†). PDAC, however, is a weaker reducing agent as compared to glycerol and the growth of AuNPs is faster when glycerol is present in the solution (Fig. 11b).
3.3. Preparation of AgNPs using crude glycerol
The efficiency of CG to reduce metal salts to respective metal nanoparticles is not limited to the formation of AuNPs. As a proof of the concept, we attempted to prepare silver nanoparticles (AgNPs) employing CG-73 as reducing agent and PVP as stabilizing agent. Fig. S14† shows the UV visible spectrum and TEM image (inset) of AgNPs prepared using CG-73 in alkaline media. The spectrum show SPR band centered at 424 nm which is characteristic of AgNPs.49 The average particle size estimated from the TEM images is around 25 nm. The number of particles increases while their size decreases with increase in pH of the reaction medium as indicated by an increased absorbance and slight blue shift of characteristic SPR band of AgNPs (Fig. S15†). Again, this can be explained based on the fact that the formation of alkoxide ions from glycerol is enhanced at higher pH.39 The result is the formation of smaller AgNPs in larger number.4. Conclusions
Gold nanoparticles with narrow size distribution were prepared using crude glycerol (CG) obtained directly from transesterification process. Our results show that CG (containing salts, moisture and a variety of fatty acids and esters), after simple filtration through a 0.2 μm syringe-fitted filter, is as effective as commercial glycerol in producing almost monodisperse AuNPs and the impurities present in CG do not affect the size distribution of the resulting AuNPs. The size distribution is principally controlled by the concentration of the stabilizing agents and glycerol itself or the trace impurities present in it seem to have no role in particle stabilization. The concentration of stabilizing agent (PVP, PDAC) is the most important factor in controlling the particle size, with final concentration of 0.4% or above being sufficient to effectively stabilize the nanoparticles. Both PVP and PDAC, besides stabilizing the AuNPs, also act as reducing agents in alkaline media, though much weaker than glycerol. The pH of the solution does not affect the particle size distribution but significantly affects the number of particles formed and the rate of their formation. This study shows that CG can be effectively employed as a low-cost reagent for the formation of metal nanoparticles.Acknowledgements
R. Parveen is thankful to The World Academy of Science (TWAS, Italy) and National Council for Scientific and Technological Development (CNPq, Brazil) for PhD fellowship and São Paulo Research Foundation (FAPESP, Brazil) for financial support. Authors also thank Dr Maria Angela T. Adorno from São Carlos School of Engineering, University of São Paulo, SP, Brazil for assistance in HPLC analysis of glycerol and Dr Janaina F. Gomes from Department of Chemical Engineering, Federal University of São Carlos, SP Brazil for her guidance and assistance at the initial stages of this work. Thanks are also due to BioBrotas Oleoquímica Industry, (Brotas, SP, Brazil) for provision of crude glycerol samples.Notes and references
- H. Kotani, R. Hanazaki, K. Ohkubo, Y. Yamada and S. Fukuzumi, Chemistry, 2011, 17, 2777–2785 CrossRef CAS PubMed.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2010, 132, 138–146 CrossRef CAS PubMed.
- S. Hebié, K. B. Kokoh, K. Servat and T. W. Napporn, Gold Bull., 2013, 46, 311–318 CrossRef.
- E. C. Dreaden, L. A. Austin, M. A. Mackey and M. A. El-Sayed, Ther. Delivery, 2012, 3, 457–478 CrossRef CAS.
- O. Bar-Ilan, R. M. Albrecht, V. E. Fako and D. Y. Furgeson, Small, 2009, 5, 1897–1910 CrossRef CAS PubMed.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CrossRef CAS PubMed.
- X. Chen, D. Zhao, Y. An, L. Shi, W. Hou and L. Chen, J. Nanopart. Res., 2009, 12, 1877–1887 CrossRef.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- A. Rajan, V. Vilas and D. Philip, J. Mol. Liq., 2015, 212, 331–339 CrossRef CAS.
- X. Chen, H. Zhu and R. Groarke, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2016, pp. 1–11 Search PubMed.
- A. Ahmad, Y. Wei, F. Syed, M. Imran, Z. U. H. Khan, K. Tahir, A. U. Khan, M. Raza, Q. Khan and Q. Yuan, RSC Adv., 2015, 5, 99364–99377 RSC.
- A. S. Sharma, H. Kaur and D. Shah, RSC Adv., 2016, 6, 28688–28727 RSC.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- F. Campanhã Vicentini, L. L. C. Garcia, L. C. S. Figueiredo-Filho, B. C. Janegitz and O. Fatibello-Filho, Enzyme Microb. Technol., 2016, 84, 17–23 CrossRef PubMed.
- R. K. Kannadorai, G. G. Y. Chiew, K. Q. Luo and Q. Liu, Cancer Lett., 2015, 357, 152–159 CrossRef CAS PubMed.
- J. Choi, J. Yang, E. Jang, J.-S. Suh, Y.-M. Huh, K. Lee and S. Haam, Anti-Cancer Agents Med. Chem., 2011, 11, 953–964 CrossRef CAS PubMed.
- M. Singh, D. C. C. Harris-Birtill, S. R. Markar, G. B. Hanna and D. S. Elson, Nanomedicine, 2015, 11, 2083–2098 CAS.
- P. Tiwari, K. Vig, V. Dennis and S. Singh, Nanomaterials, 2011, 1, 31–63 CrossRef CAS.
- E. Hutter and D. Maysinger, Microsc. Res. Tech., 2011, 74, 592–604 CrossRef CAS PubMed.
- P. C. Chen, S. C. Mwakwari and A. K. Oyelere, Nanotechnol., Sci. Appl., 2008, 1, 45–65 CAS.
- J. Han, Y. Liu and R. Guo, Adv. Funct. Mater., 2009, 19, 1112–1117 CrossRef CAS.
- S.-A. Dong and S.-P. Zhou, J. Mater. Sci. Eng. B, 2007, 140, 153–159 CrossRef CAS.
- S. Boufi, M. R. Vilar, A. M. Ferraria and A. M. Botelho do Rego, Colloids Surf., A, 2013, 439, 151–158 CrossRef CAS.
- S. Gudlur, C. Sandén, P. Matoušková, C. Fasciani and D. Aili, J. Colloid Interface Sci., 2015, 456, 206–209 CrossRef CAS PubMed.
- H. Ma, B. Yin, S. Wang, Y. Jiao, W. Pan, S. Huang, S. Chen and F. Meng, ChemPhysChem, 2004, 5, 68–75 CrossRef CAS PubMed.
- D. Zhang and P. Diao, J. Electroanal. Chem., 2015, 755, 174–181 CrossRef CAS.
- M. Tsuji, M. Hashimoto, Y. Nishizawa, M. Kubokawa and T. Tsuji, Chem.–Eur. J., 2005, 11, 440–452 CrossRef CAS PubMed.
- P. Nalawade, T. Mukherjee and S. Kapoor, Adv. Nanoparticles, 2013, 02, 78–86 CrossRef.
- A. N. Grace and K. Pandian, Colloids Surf., A, 2006, 290, 138–142 CrossRef CAS.
- R. Genc, G. Clergeaud, M. Ortiz and C. K. O'Sullivan, Langmuir, 2011, 27, 10894–10900 CrossRef CAS PubMed.
- J. Yu, D. Xu, H. N. Guan, C. Wang, L. K. Huang and D. F. Chi, Mater. Lett., 2016, 166, 110–112 CrossRef CAS.
- R. Mata, A. Bhaskaran and S. R. Sadras, Particuology, 2016, 24, 78–86 CrossRef CAS.
- U. Shedbalkar, R. Singh, S. Wadhwani, S. Gaidhani and B. A. Chopade, Adv. Colloid Interface Sci., 2014, 209, 40–48 CrossRef CAS PubMed.
- K. B. Narayanan and N. Sakthivel, Adv. Colloid Interface Sci., 2010, 156, 1–13 CrossRef CAS PubMed.
- R. Parveen, J. F. Gomes, S. Ullah, J. J. S. Acuña and G. Tremiliosi-Filho, J. Nanopart. Res., 2015, 17, 418 CrossRef.
- A. G. Garcia, P. P. Lopes, J. F. Gomes, C. Pires, E. B. Ferreira, R. G. M. Lucena, L. H. S. Gasparotto and G. Tremiliosi-Filho, New J. Chem., 2014, 38, 2865–2873 RSC.
- L. H. S. Gasparotto, A. C. Garcia, J. F. Gomes and G. Tremiliosi-Filho, J. Power Sources, 2012, 218, 73–78 CrossRef CAS.
- A. C. Garcia, L. H. S. Gasparotto, J. F. Gomes and G. Tremiliosi-Filho, Electrocatalysis, 2012, 3, 147–152 CrossRef CAS.
- J. F. Gomes, A. C. Garcia, E. B. Ferreira, C. Pires, V. L. Oliveira, G. Tremiliosi-Filho and L. H. S. Gasparotto, Phys. Chem. Chem. Phys., 2015, 17, 21683–21693 RSC.
- R. Genç, M. Ortiz and C. K. O'Sullivan, Langmuir, 2009, 25, 12604–12613 CrossRef PubMed.
- E. B. Ferreira, J. F. Gomes, G. Tremiliosi-Filho and L. H. S. Gasparotto, Mater. Res. Bull., 2014, 55, 131–136 CrossRef CAS.
- C.-H. C. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. M. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- M. Ayoub and A. Z. Abdullah, Renewable Sustainable Energy Rev., 2012, 16, 2671–2686 CrossRef CAS.
- H. W. Tan, A. R. Abdul Aziz and M. K. Aroua, Renewable Sustainable Energy Rev., 2013, 27, 118–127 CrossRef CAS.
- M.-S. Cui, J. Deng, X.-L. Li and Y. Fu, ACS Sustainable Chem. Eng., 2016, 4, 1707–1714 CrossRef CAS.
- C. E. Hoppe, M. Lazzari, I. Pardiñas-Blanco and M. A. López-Quintela, Langmuir, 2006, 22, 7027–7034 CrossRef CAS PubMed.
- M. Zhou, B. Wang, Z. Rozynek, Z. Xie, J. O. Fossum, X. Yu and S. Raaen, Nanotechnology, 2009, 20, 505606 CrossRef PubMed.
- M. Habib Ullah, T. Hossain and C.-S. Ha, J. Mater. Sci., 2011, 46, 6988–6997 CrossRef.
- H. Wang, X. Qiao, J. Chen, X. Wang and S. Ding, Mater. Chem. Phys., 2005, 94, 449–453 CrossRef CAS.
- C. Kan, W. Cai, C. Li and L. Zhang, J. Mater. Res., 2005, 20, 320–324 CrossRef CAS.
- H. Chen, Y. Wang, Y. Wang, S. Dong and E. Wang, Polymer, 2006, 47, 763–766 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 13C-NMR, GC-MS, FTIR analysis data of crude glycerol, DLS data on particle size. See DOI: 10.1039/c6ra14259a |
| This journal is © The Royal Society of Chemistry 2016 |