Vinyl sulfone-activated silica for efficient covalent immobilization of alkaline unstable enzymes: application to levansucrase for fructooligosaccharide synthesis
P. Santos-Morianoa,
L. Monsalve-Ledesmaa,
M. Ortega-Muñozb,
L. Fernandez-Arrojoa,
A. O. Ballesterosa,
F. Santoyo-Gonzalezb and
F. J. Plou*a
aInstituto de Catalisis y Petroleoquimica, CSIC, Marie Curie 2, 28049 Madrid, Spain. E-mail: fplou@icp.csic.es
bDepartamento de Química Orgánica, Universidad de Granada, Spain
First published on 29th June 2016
Abstract
Most methodologies for covalent immobilization of enzymes usually take place at high pH values to enhance the nucleophilicity of protein reactive residues; however, many enzymes inactivate during the immobilization process due to their intrinsic instability at alkaline pH values. Vinyl sulfone (VS)-activated carriers may react with several protein side-chains at neutral pHs. In this work, levansucrase-an alkaline unstable enzyme of technological interest because it forms fructooligosaccharides (FOS) and levan from sucrose-was covalently attached to VS-activated silica at pH 7.0 in a short time (5 h). Theoretical immobilization yields were close to 95% but the apparent activity did not surpass 25%, probably due to random attachment with unproductive orientations and rigidification of the enzyme structure. Due to diffusional hindrance and/or local microenvironmental effects caused by the silica surface, the immobilized levansucrase was unable to produce levan but synthesized a similar amount of FOS than the free enzyme [95 g L−1 in 28 h, with a major contribution of FOS of the β(2 → 1) type]. The VS-activated biocatalysts showed a notable operational stability in batch reactors.
1. Introduction
The design of efficient and reproducible immobilization strategies is one of the main hurdles that hinder the implementation of more industrial-scale enzymatic processes.1 Immobilization allows easy separation and reuse of the biocatalyst, facilitates the recovery of products and usually stabilizes enzymes against temperature, extreme pH or other denaturant agents.2 Covalent binding of enzymes to pre-activated supports is preferred over simple adsorption because it gives rise to strong and stable linkages between the biomolecule and the carrier that result in robust biocatalysts with minimal enzyme leakage. This fact is particularly crucial in biotransformations that take place in aqueous media and involve polar substrates – such as carbohydrates – in which protein molecules can be progressively desorbed.3 Different materials, e.g. polysaccharides (agarose), organic polymers (polymethacrylate) or porous silica, can be chemically activated with reactive groups (glyoxal, epoxy, hydroxy-succinimide, ethyl dimethylaminopropyl carbodiimide, etc.) to covalently attach enzymes. Most of the coupling protocols must be carried out at high pH values in order to enhance the nucleophilicity of the involved residues in the protein, typically amine (lysines), thiol (cysteines) and/or phenoxyl (tyrosines); in many cases, the enzymes are not alkaline stable, which leads to the inactivation of the biocatalyst during the immobilization process.The activation of carriers with divinyl sulfone (DVS) for enzyme immobilization was first described by Bryjak et al.4 for an organic support (cellulose) and by Ortega-Muñoz et al.,5,6 for an inorganic material (silica). This approach has been applied to other supports such as agarose.7,8 VS-activated carriers enable covalent coupling at neutral pH, and have been reported to immobilize several enzymes and proteins even under moderate acidic conditions.5,7–9 The covalent bonds between the enzyme and the support are formed by means of a Michael-type addition that involves the amino groups of lysines, the imidazole moieties of histidines and/or the sulfhydryl group of cysteines, as represented in Scheme 1.
Levansucrases (EC 2.4.1.10) are fructosyltransferases that produce fructooligosaccharides (FOS) of formula GFn, (with n ranging from 1 to 10), which are formed by sequential transfructosylation of a fructosyl moiety to a molecule of sucrose. The linkages between fructoses in the FOS synthesized by levansucrase are mainly of the β(2 → 6) type, thus yielding 6-kestose and other FOS of the 6F-series.
Long chains of fructoses coupled by β(2 → 6) bonds render the polysaccharide levan, that exhibits several interesting bioactivities.10 FOS show low values of caloric intake (2 kcal g−1) and glycemic index, and are used as food ingredients due to their prebiotic properties – favouring the development of Bifidobacteria and Lactobacillus – as well as other side effects such as a reduced risk of suffering colon cancer, an improved gut absorption of Ca2+ and Mg2+, and a reduction of blood lipid levels.11
The production of FOS vs. levan by levansucrases strongly depends on the reaction conditions, mainly substrate concentration and temperature.12 In our previous studies with the levansucrase from Zymomonas mobilis (LEV), we also observed the production of FOS of the 1F-series (e.g. 1-kestose) and 6G-series (e.g. neokestose), as well as other carbohydrates such as blastose.12 Moreover, the hydrolytic activity of levansucrases gives rise to the formation of free fructose; this monosaccharide can also accept fructosyl moieties forming levan oligomers (levanbiose, levantriose, etc.).13,14
The immobilization of levansucrase from Z. mobilis has been previously reported, mainly by adsorption on different materials such as magnetite,15 chitin16 or hydroxyapatite.17,18 In our laboratory, we immobilized levansucrase by entrapment in alginate beads followed by further dehydration for the formation of DALGEEs (Dry ALGinate Entrapped Enzymes),19 after crosslinking with transglutaminase to minimize the leakage of the enzyme.20 However, the covalent immobilization of levansucrase still remains a challenge, which is related to the intrinsic low stability of the enzyme at alkaline pH.
In this work, the covalent immobilization of Z. mobilis levansucrase onto vinyl sulfone-activated silica particles was attempted at neutral pH. In order to evaluate the potential of the immobilized biocatalysts at industrial scale, the operational stability in a batch reactor for FOS production was assessed for the first time with these supports.
2. Experimental
2.1. Materials
Levansucrase (LEV-Y) from Z. mobilis was kindly donated by Amano (Japan) as a powder preparation. Sucrose, fructose and glucose were from Merck. 1-Kestose and nystose were from TCI Europe. 1F-Fructosylnystose was from Megazyme. 6-Kestose, blastose, neokestose, neonystose and 6,6-nystose were previously purified in the laboratory.12,21–23 VS activated silica carriers were prepared as described elsewhere.62.2. Preparation of VS-activated silica
Activated silica gel (5 g) was suspended in dried toluene (25 mL) and then [3-(methylamino)propyl]-trimethoxysilane (1.25 g) was added. The reaction mixture was heated under reflux for 2 h. Evaporation of the solvent up to half of the volume to remove the formed methanol was followed by further addition of dry toluene (25 mL) and additional reflux for one hour. The reaction mixture was filtered and the white powder washed with dichloromethane (4 × 20 mL) and dried under vacuum (1 mm Hg) at 50 °C for 16 h giving the amine functionalized silica (5.7 g). For the preparation of vinyl sulfone silica (N-type), the amine derivatized silica (3.13 g) was suspended in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol (1
2-propanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 10 mL) and then divinyl sulfone (DVS, 1.4 mL) was added. The resulting suspension was stirred overnight at room temperature. The reaction mixture was filtered and the white powder washed with methanol (3 × 20 mL) and dried under vacuum (1 mm Hg) at 50 °C for 16 h giving the vinyl sulfone silica (3.33 g).
2, 10 mL) and then divinyl sulfone (DVS, 1.4 mL) was added. The resulting suspension was stirred overnight at room temperature. The reaction mixture was filtered and the white powder washed with methanol (3 × 20 mL) and dried under vacuum (1 mm Hg) at 50 °C for 16 h giving the vinyl sulfone silica (3.33 g).
2.3. Characterization of VS-activated silica
Nitrogen isotherms were performed at the temperature of liquid N2 (−196 °C), using a Micromeritics ASAP 2420 device. The samples were previously degassed at 80 °C for 16 h to a residual vacuum of 5 × 10−3 Torr, to remove any loosely-held adsorbed species. The surface area of the support was determined by the BET method.24 The distribution of pore sizes was established from the adsorption branch of the isotherm using the BJH model Scanning electron microscopy (SEM) was performed using an Hitachi S-3000N microscope on samples previously metallized with gold in a sputter Quorum, model Q150T-S.2.4. Immobilization of levansucrase on VS activated carriers
A micro-scale procedure3 using micro-centrifuge filter tubes (Spin-X®, 0.45 μm, cellulose acetate, Corning Inc., USA) was employed for the immobilization of levansucrase on VS-activated silica carriers. The enzyme was incubated with the support at two different ratios (w/w): 10 and 100 mg of protein per gram of carrier. Levansucrase was previously dissolved in 50 mM potassium phosphate buffer (pH 7.0). The immobilization filter tubes were incubated for 5 h at room temperature with mild agitation. The solid biocatalyst (LEV-VS) was separated of the remaining solution by centrifugation and washed thoroughly with 50 mM sodium acetate buffer (pH 5.4). The immobilization yield was determined by subtracting the total protein and enzymatic activity in the filtrate and the washing solutions from the values obtained with the starting enzyme solution.2.5. Standard activity assay
The activity of levansucrase was determined using 100 g L−1 sucrose in 50 mM sodium acetate buffer (pH 5.4) as substrate following the release of reducing sugars in 20 min by the 3,5-dinitrosalicylic acid (DNS) assay adapted to 96-well plates.25 One unit of activity (U) was defined as that corresponding to the release of one μmol of reducing sugar per minute. For the measurement of the apparent activity of the immobilized biocatalysts, a method developed in our laboratory, which involves the use of filtered micro-centrifuge tubes, was used.3 Essentially, 20 mg of biocatalyst and 100 g sucrose per l in working buffer (50 mM sodium acetate buffer, pH 5.4) were incubated in a micro-centrifuge filter tube (Spin-X®, 0.45 μm) containing a filter of cellulose acetate. The mixture was incubated at 40 °C for 20 min with vigorous (900 rpm) stirring. The reaction mixture was separated from the biocatalyst by centrifugation at 5000 × g. To inactivate the possible lixiviated enzyme, 0.4 M Na2CO3 was added to the supernatant. Then, 50 μL (conveniently diluted) were transferred in triplicate to the wells of a microplate, and the concentration of reducing sugars was measured by the standard DNS assay. Total protein concentration was determined by the Bradford assay using the Bio-Rad dye reagent.2.6. Enzyme stability
For the analysis of pH stability, the enzyme was incubated in 50 mM Britton & Robinson (B&R, acetate–phosphate–borate) buffer26 at different pH values (2.0–9.0) and 30 °C for 24 h, and the activity with 100 g L−1 sucrose was measured by the DNS assay at 40 °C as described. For the determination of thermostability, the enzyme was incubated at different temperatures (30–80 °C) for 24 h in 50 mM sodium acetate buffer (pH 5.4) in a thermocycler (BioRad) using PCR microplates. The residual activity was measured by the activity assay under standard conditions (40 °C, pH 5.4). All the experiments were performed in triplicate and the error was expressed as the standard deviation of the three measurements.2.7. Operational stability of immobilized biocatalysts
Operational stability in batch was assayed following a methodology recently described by our group.3 A known amount of LEV-VS was placed in filtered micro-centrifuge tubes (Spin-X®, 0.45 μm) per duplicate and a solution of 100 g L−1 sucrose was added. Reaction was carried out at the optimal conditions for the soluble enzyme (40 °C, pH 5.4) for 20 min. The reaction was stopped by separating the biocatalyst from the solution by centrifugation at 5000 × g. The amount of reducing sugars in the supernatant was measured by the DNS method. Between cycles, the biocatalyst was washed three times with 50 mM sodium acetate buffer, pH 5.4.2.8. Production of FOS by immobilized biocatalysts
To a solution of 600 g L−1 sucrose in 50 mM sodium acetate buffer pH 5.4, LEV-VS was added to reach a final activity of 5 U mL−1. The reaction was incubated at 40 °C in a roller stirrer, and samples were taken at intervals and inactivated with 0.4 M Na2CO3. The identification and quantification of FOS was carried out by High Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD, Dionex ICS3000 system) and a CarboPack PA1 column (4 × 250 mm) connected to a PA-1 guard column. Initial mobile phase was 20 mM NaOH at 1 mL min−1 and it was maintained for 13 min. Then a gradient from 20 to 100 mM NaOH and from 0 to 40 mM sodium acetate was performed in 7 min. These conditions were kept for 10 min and then sodium acetate concentration was increased from 40 to 100 mM in 5 min and maintained for 2 min. All eluents were degassed by flushing with helium. The peaks were analyzed using Chromeleon software.3. Results and discussion
3.1. Temperature and pH stability of soluble levansucrase
The thermal and pH stability of levansucrase was studied in order to analyze the optimal immobilization conditions for this enzyme. After 24 h incubations, the residual activity was determined by the standard activity assay. We observed that the enzyme was stable at temperatures between 4 and 45 °C and pH 3–7 (Fig. 1). The complete loss of activity at pH ≥ 8.0 indicates that this enzyme is not appropriate for covalent immobilization at alkaline pH by typical methodologies. On the contrary, one of the main advantages of the VS-activated supports is that covalent bonds between the carrier and the enzyme can be formed at neutral pH. Levansucrase keeps 60% of its initial activity after incubation at pH 7.0 for 24 h (Fig. 1); therefore, immobilization under these conditions with VS-activated silica carriers should not drastically decrease the activity.It is worth noting that the enzyme was slightly activated upon incubation at 35 °C and also at pH 4.0. In the last case, activation could be related to the formation of ordered microfibril structures at acid pH as described by Goldman et al.27 Neutral pH and room temperature were selected as the immobilization conditions.
3.2. Characterization of vinyl sulfone-activated silica
Silica was selected as carrier due to its extraordinary chemical, mechanical and biological stability, as well as its resistance to large changes in pressure and flow-rates in different reactors.5 Activation of silica with DVS was carried out as described in the Experimental section.6 The morphology and porous structure of VS-activated silica is illustrated in the Scanning Electron Microscopy pictures (Fig. 2). The picture at 100× (Fig. 2A) shows amorphous particles with an average size of 100 μm. The particles have not visible porous at 1000× (Fig. 2B).Table 1 summarizes the main textural properties of VS-activated silica. Fig. 3 represents the pore size distribution of this support calculated using the BJH method.24 VS-activated silica is basically a mesoporous material, with an average pore size close to 7 nm. The contribution of the micropore region was negligible. Considering that this enzyme is about 5 × 6 × 7 nm in diameter (see 3D model in Fig. 4), the above data indicates that levansucrase can hardly diffuse into the pores, and the enzyme must be basically immobilized on the external surface of the silica particles.
| BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|
| 242 | 0.36 | 6.8 |
 | ||
| Fig. 3 BJH adsorption cumulative pore volume (solid black) and pore distribution (dash red) of vinyl sulfone-activated silica. | ||
3.3. Immobilization of levansucrase on VS-activated silica
Levansucrase was immobilized on VS-activated silica carriers at pH 7.0 using two different protein loadings: 10 mg g−1 and 100 mg g−1 support. Under the best conditions (10 mg g−1), the theoretical yield, calculated by measuring the remaining protein and activity in the filtrate and washing solutions, was 88% for protein and 95% for activity (Table 2). These results indicated that it was possible to successfully attach, at neutral pH, an enzyme that is intrinsically unstable at alkaline pH. The higher yield in activity than in protein indicated that levansucrase was able to bind to the carrier slightly better than other proteins present in the preparation. It is worth noting that covalent immobilization on VS-activated silica was completed in only 5 h; it is a significantly shorter time than the required for other materials such as epoxy-activated supports (48–72 h).28| Protein | Activity | |||||
|---|---|---|---|---|---|---|
| Initial loading (mg g−1) | Yielda (%) | Initial (U) | Theoreticala | Apparentb | ||
| Activity (U g−1) | Yield (%) | Activity (U g−1) | Yield (%) | |||
| a Theoretical values determined by subtracting the total initial protein or activity prior to immobilization and the remaining protein or activity in the filtrate and washing solutions after immobilization.b Experimental activity (standard assay). | ||||||
| 10 | 89 ± 4 | 8.0 ± 0.5 | 152 ± 11 | 96 ± 13 | 40 ± 15 | 25 ± 9 |
| 100 | 83 ± 4 | 133 ± 04 | 2500 ± 100 | 93 ± 7 | 185 ± 5 | 7 ± 0.2 |
Other covalent immobilization methods that can be carried out at neutral pH are characterized by a lower efficiency than this one involving vinyl sulfone activation. Glutaraldehyde-assisted strategies are also very fast but the high reactivity of this molecule usually causes a notable loss of activity. Methods that involve carboxylic acids of the protein typically employ the carbodiimide chemistry, followed by reaction with nucleophiles on the surface of the carrier.29 Such protocols often lead to a low recovery of activity, partly because carboxyl groups are essential for the catalytic machinery of numerous enzymes. In addition, the low stability of O-acylisourea and N-hydroxysuccinimide ester intermediates in aqueous solution contribute to the modest coupling efficiency.30
It is well known that enzyme reactivity depends on the number and accessibility of reactive groups. The levansucrase from Z. mobilis contains 14 lysines, 9 histidines and 3 free cysteines in its amino acid sequence (GenBank: AAA27702.1). With this sequence, modelling of the 3D structure of levansucrase from Z. mobilis was performed with Swissmodel, using the 3D structure of levansucrase from B. subtilis (PDB: 1OYG, 24% identity) as a template. The model shows that most of the Lys (7 residues) and several histidines are located on the enzyme surface ready to react with the vinyl sulfone groups of the carrier (Fig. 4), which could explain the efficient coupling.
Although the theoretical immobilization yields were quite high, the apparent activities (experimental) of the immobilized biocatalysts LEV-VS, measured by the DNS method, were 185 U g−1 (100 mg g−1) and 40 U g−1 (10 mg g−1), with apparent yields of 7% and 25%, respectively. The apparent activity is affected by mass transfer and diffusional limitations,31 typically causing a decrease of the activity displayed by the enzyme. However, the small pore size of VS-activated silica seems to exclude mass diffusion constraints in this case. However, the presence of Lys or His residues next to the catalytic center could lead to the binding of the enzyme in an unproductive way. In addition, the rigidification of the enzyme caused by the formation of covalent bonds between the enzyme and the support could also contribute to a decrease in activity.
This loss of activity upon immobilization was in accordance with the kinetic parameters of the soluble and immobilized enzymes measured under the standard conditions with sucrose. The KM values for free and covalently-attached enzymes were 100 ± 11 and 87 ± 12 mM, respectively, indicating that the affinity of immobilized enzyme for sucrose is hardly affected. In contrast, Vmax decreased from 1265 ± 45 to 117 ± 4 μmol mg−1 min−1 as a consequence of immobilization. These values indicate that the insolubilization of the enzyme mainly affects to the rate of transformation of substrate into products.
3.4. Operational stability of LEV-VS in batch reactions
In order to assess the operational stability of the immobilized biocatalyst, LEV-VS was used in a series of batch reactions with 100 g L−1 sucrose as substrate. After each reaction cycle (20 min), the biocatalyst was thoroughly washed with buffer to remove residual substrate and products, as well as any desorbed enzyme. After 17 cycles, LEV-SV conserved around 50% of its initial activity (Fig. 5). After an approximate 40% loss of activity in the first 4 cycles, the activity of LEV-VS remained quite stable indicating that the biocatalyst can be suitable for its repeated use in batch reactors. This loss of activity in the first cycles is typical of covalent immobilizations studies,32–34 and can be a consequence of the desorption of enzyme molecules that are non-covalently attached to the carrier – which is favoured by the polarity of sugar solutions – or the existence of different populations of enzyme molecules differing in the number of covalent bonds with the support matrix and thus in their intrinsic stability.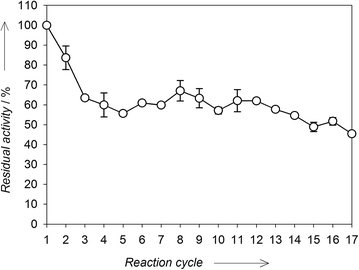 | ||
| Fig. 5 Operational stability of LEV-VS after a series of reactions in batch. Values are referred to the activity of LEV-VS in the first cycle. | ||
3.5. Production of fructooligosaccharides with LEV-VS
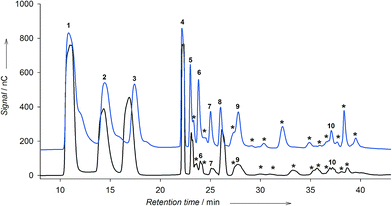
The production of fructooligosaccharides and levan by LEV-VS was compared with that of the free enzyme. In the case of soluble levansucrase, we previously observed that depending on the reaction conditions – mainly temperature and substrate concentration – the enzyme produced preferably levan or FOS.12 At low temperature and low concentration of sucrose, the enzyme synthesized levan in detriment of FOS. On the other hand, using 600 g L−1 sucrose at 40 °C, the enzyme produced FOS with different linkages (Fig. 6, blue chromatogram).Even under the optimal conditions for levan formation, the immobilized levansucrase on vinyl sulfone did not synthesize levan (data not shown). On the contrary, the enzyme was able to produce FOS. The formation of FOS by LEV-VS was followed by HPAEC-PAD under the optimal conditions for oligosaccharide synthesis (600 g L−1 sucrose and 40 °C), and compared with the soluble enzyme (Fig. 6). Comparing both chromatograms, it can be stated that the reaction with the immobilized levansucrase is more selective, yielding fewer products than with the soluble enzyme. The soluble levansucrase was able to form FOS of the 1F-(inulin-like), 6F-(6-kestose family) and 6G-(neoFOS) series, as well as several unidentified peaks (Fig. 6, asterisks). On the other hand, LEV-VS is more selective towards the formation of FOS of the β(2 → 1) type, i.e. 1-kestose and nystose (Fig. 6, black chromatogram, peaks 4 and 8). This fact could help to explain the negligible levan formation, which would require the production of an homologous series of FOS of the β(2 → 6) family. The diffusional restrictions within the immobilized biocatalyst could also favor the formation of small oligomers in comparison with long polysaccharides. It cannot be excluded that the local microenvironment on the surface of the activated silica may exert an effect on the selectivity of the process, as reported with other immobilized enzymes.35 In fact, a change in the product profile of levansucrase upon immobilization was observed in a previous work using the alginate entrapment approach.20
The main products synthesized by soluble and immobilized levansucrases were quantified (Fig. 7). Starting with 600 g L−1 sucrose, the immobilized enzyme rendered a maximum yield of approximately 95 g L−1 of identified FOS after 28 h (Fig. 7A), a value very similar to that obtained with the soluble enzyme, under the same conditions. However, the distribution of the main products varies between both preparations (Fig. 7B), as the syrup obtained with the immobilized enzyme was enriched in 1-kestose and nystose, whereas the soluble enzyme gave rise to a more heterogeneous mixture. Considering the mass balance, the rest of products must correspond to unidentified FOS and, in the case of the soluble enzyme, to a small amount of levan.12
The transfructosylation to hydrolysis ratio (T/H) refers to the preference of the enzyme to transfer the fructose moiety from sucrose to another sugar – transfructosylation – or to a molecule of water – hydrolysis. The hydrolytic activity (H) was quantified on the basis of the concentration of free fructose. The transfructosylation activity (T) was calculated by subtracting the concentration of free glucose and free fructose. The T/H ratio was calculated dividing both activities. Sucrose, fructose and glucose concentrations were measured after 28 h in order to obtain those values for both the free and the immobilized enzyme. The T/H ratio slightly increased upon immobilization (1.7 vs. 1.2), probably due to a change in the microenvironment around the enzyme molecules.
4. Conclusions
The levansucrase from Z. mobilis is a typical case of an enzyme with great biotechnological potential that cannot be covalently immobilized by most of the described methodologies because it is rapidly inactivated at alkaline pH. Vinyl sulfone-activated silica was successfully employed to immobilize levansucrase at neutral pH thus circumventing such limitations. In addition, silica offers high chemical, physical and biological stability. The apparent activity (experimental) of the best immobilized biocatalyst was close to 100 U g−1. However, not all the enzyme molecules were linked to the carrier in a productive way, because different lysine, histidine and cysteine residues can be involved in the binding process resulting eventually in unproductive orientations. The immobilized levansucrase was not able to produce the polysaccharide levan – probably due to steric restrictions and effects of the microenvironment – but synthesized similar amounts of FOS than the free enzyme (95 g L−1 after 28 h, under the optimal conditions). The immobilized biocatalysts displayed significant reuse stability in batch reactions; however, further studies in continuous stirred-tank and fixed-bed reactors will be of great value to evaluate the potential of these biocatalysts.Acknowledgements
We thank Dr Javier Agundez for technical help in the characterization of the support. This work was supported by a grant from the Spanish Ministry of Economy and Competitiveness (BIO2013-48779-C4-1-R). We thank the support of COST-Action CM1303 on Systems Biocatalysts. P. S.-M. thanks the Spanish Ministry of Education for FPU Grant (FPU13/01185).References
- R. A. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- P. Torres-Salas, A. del Monte-Martinez, B. Cutiño-Avila, B. Rodriguez-Colinas, M. Alcalde, A. O. Ballesteros and F. J. Plou, Adv. Mater., 2011, 23, 5275–5282 CrossRef CAS PubMed.
- L. Fernandez-Arrojo, P. Santos-Moriano, B. Rodriguez-Colinas, A. O. Ballesteros and F. J. Plou, Biotechnol. Lett., 2015, 37, 1593–1600 CrossRef CAS PubMed.
- J. Bryjak, J. Liesiene and B. N. Kolarz, Colloids Surf., B, 2008, 61, 66–74 CrossRef CAS PubMed.
- M. Ortega-Muñoz, J. Morales-Sanfrutos, A. Megia-Fernandez, F. J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, J. Mater. Chem., 2010, 20, 7189–7196 RSC.
- J. Morales-Sanfrutos, J. Lopez-Jaramillo, M. Ortega-Muñoz, A. Megia-Fernandez, F. Perez-Balderas, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Org. Biomol. Chem., 2010, 8, 667–675 CAS.
- J. C. S. dos Santos, N. Rueda, R. Torres, O. Barbosa, L. R. B. Gonçalves and R. Fernandez-Lafuente, Process Biochem., 2015, 50, 918–927 CrossRef CAS.
- J. C. S. dos Santos, N. Rueda, O. Barbosa, M. d. C. Millan-Linares, J. Pedroche, M. del Mar Yuste, L. R. B. Gonçalves and R. Fernandez-Lafuente, J. Mol. Catal. B: Enzym., 2015, 117, 38–44 CrossRef CAS.
- F. J. Lopez-Jaramillo, M. Ortega-Muñoz, A. Megia-Fernandez, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Bioconjugate Chem., 2012, 23, 846–855 CrossRef CAS PubMed.
- R. Srikanth, C. H. S. S. S. Reddy, G. Siddartha, M. J. Ramaiah and K. B. Uppuluri, Carbohydr. Polym., 2015, 120, 102–114 CrossRef CAS PubMed.
- D. A. Flores-Maltos, S. I. Mussatto, J. C. Contreras-Esquivel, R. Rodriguez-Herrera, J. A. Teixeira and C. N. Aguilar, Crit. Rev. Biotechnol., 2016, 36, 259–267 CrossRef CAS PubMed.
- P. Santos-Moriano, L. Fernandez-Arrojo, A. Poveda, J. Jimenez-Barbero, A. O. Ballesteros and F. J. Plou, J. Mol. Catal. B: Enzym., 2015, 119, 18–25 CrossRef CAS.
- K.-H. Jang, E.-J. Ryu, B.-S. Park, K.-B. Song, S. A. Kang, C. H. Kim, T.-B. Uhm, Y.-I. Park and S.-K. Rhee, J. Agric. Food Chem., 2003, 51, 2635–2636 CrossRef PubMed.
- L. Mendez-Lorenzo, J. R. Porras-Dominguez, E. Raga-Carbajal, C. Olvera, M. E. Rodriguez-Alegria, E. Carrillo-Nava, M. Costas and A. Lopez Munguia, PLoS One, 2015, 10, e0143394 Search PubMed.
- K.-H. Jang, K.-B. Song, B.-S. Park, C. H. Kim, B. H. Chung, R. W. Choue, K. S. Lee, C. Lee, U.-H. Chun and S.-K. Rhee, Process Biochem., 2001, 37, 339–343 CrossRef CAS.
- C. J. Chiang, J. Y. Wang, P. T. Chen and Y. P. Chao, Appl. Microbiol. Biotechnol., 2009, 82, 445–451 CrossRef CAS PubMed.
- R. Chambert and M.-F. Petit-Glatron, Carbohydr. Res., 1993, 244, 129–136 CrossRef CAS PubMed.
- H. K. Jang, B. K. Song, S. J. Kim, H. C. Kim, H. B. Chung and K. S. Rhee, Bioprocess Eng., 2000, 23, 89–93 CrossRef.
- L. Fernandez-Arrojo, B. Rodriguez-Colinas, P. Gutierrez-Alonso, M. Fernandez-Lobato, M. Alcalde, A. O. Ballesteros and F. J. Plou, Process Biochem., 2013, 48, 677–682 CrossRef CAS.
- P. Santos-Moriano, L. Fernández-Arrojo, B. Rodriguez-Colinas, A. Ballesteros and F. J. Plou, An. R. Acad. Nac. Farm., 2015, 81, 48–62 Search PubMed.
- M. Alvaro-Benito, M. De Abreu, L. Fernandez-Arrojo, F. J. Plou, J. Jimenez-Barbero, A. Ballesteros, J. Polaina and M. Fernandez-Lobato, J. Biotechnol., 2007, 132, 75–81 CrossRef CAS PubMed.
- D. Linde, B. Rodriguez-Colinas, M. Estevez, A. Poveda, F. J. Plou and M. Fernandez Lobato, Bioresour. Technol., 2012, 109, 123–130 CrossRef CAS PubMed.
- P. Zambelli, L. Fernandez-Arrojo, D. Romano, P. Santos-Moriano, M. Gimeno-Perez, A. Poveda, R. Gandolfi, M. Fernandez-Lobato, F. Molinari and F. J. Plou, Process Biochem., 2014, 49, 2174–2180 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- I. Ghazi, L. Fernandez-Arrojo, H. Garcia-Arellano, M. Ferrer, A. Ballesteros and F. J. Plou, J. Biotechnol., 2007, 128, 204–211 CrossRef CAS PubMed.
- H. T. S. Britton and R. A. Robinson, J. Chem. Soc., 1931, 1456–1462, 10.1039/jr9310001456.
- D. Goldman, N. Lavid, A. Schwartz, G. Shoham, D. Danino and Y. Shoham, J. Biol. Chem., 2008, 283, 32209–32217 CrossRef CAS PubMed.
- J. Berrio, F. J. Plou, A. Ballesteros, A. T. Martinez and M. J. Martinez, Biocatal. Biotransform., 2007, 25, 130–134 CrossRef CAS.
- Y. Gao and I. Kyratzis, Bioconjugate Chem., 2008, 19, 1945–1950 CrossRef CAS PubMed.
- A. A. Homaei, R. Sariri, F. Vianello and R. Stevanato, J. Chem. Biol., 2013, 6, 185–205 CrossRef PubMed.
- P. J. Worsfold, Pure Appl. Chem., 1995, 67, 597–600 CrossRef CAS.
- M. T. Martin, F. J. Plou, M. Alcalde and A. Ballesteros, J. Mol. Catal. B: Enzym., 2003, 21, 299–308 CrossRef CAS.
- R. M. Azevedo, J. B. Costa, P. Serp, J. M. Loureiro, J. L. Faria, C. G. Silva and A. P. M. Tavares, J. Chem. Technol. Biotechnol., 2015, 90, 1570–1578 CrossRef CAS.
- B. Binay, D. Alagöz, D. Yildirim, A. Çelik and S. S. Tükel, Beilstein J. Org. Chem., 2016, 12, 271–277 CrossRef CAS PubMed.
- R. C. Rodrigues, C. Ortiz, A. Berenguer-Murcia, R. Torres and R. Fernández-Lafuente, Chem. Soc. Rev., 2013, 42, 6290–6307 RSC.
| This journal is © The Royal Society of Chemistry 2016 |