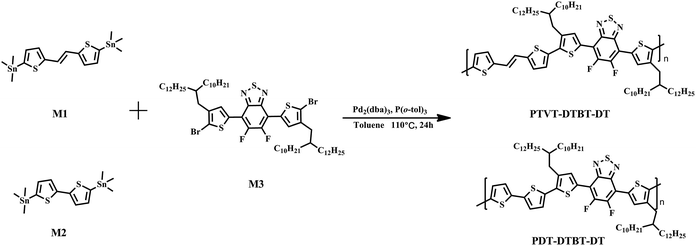
(E)-1,2-Di(thiophen-2-yl)ethene based high mobility polymer for efficient photovoltaic devices without any post treatment†
Junzhen Ren‡
a,
Yongchao Zhang‡b,
Fushuai Liua,
Yan Yanc,
Meng Qiub,
V. A. L. Royc,
Huilin Zhenga,
Mingliang Sun*a and
Renqiang Yang*b
aInstitute of Material Science and Engineering, Ocean University of China, Qingdao 266100, People's Republic of China. E-mail: mlsun@ouc.edu.cn
bCAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, People's Republic of China. E-mail: yangrq@qibebt.ac.cn
cCenter of Super-Diamond and Advanced Films (COSDAF), City University of Hong Kong, Hong Kong, China
First published on 11th July 2016
Abstract
In order to investigate the effect of an (E)-1,2-di(thiophen-2-yl)ethene (TVT) unit on the hole mobility and photovoltaic properties of dithienyl-difluorobenzothiadiazole (DTBT) based polymers, two conjugated polymers PDT-DTBT-DT (thiophene backboned) and PTVT-DTBT-DT (TVT backboned) were synthesized. Compared to PDT-DTBT-DT, the backbone conformation of PTVT-DTBT-DT could be well modulated by the TVT unit, leading to an extended conjugation length and strengthened intermolecular interaction. Interestingly, it's found that the ultraviolet-visible (UV-vis) absorption peaks of the PTVT-DTBT-DT film was blue-shifted compared to that of the solution. The organic field-effect transistor (OFETs) characterization showed that PTVT-DTBT-DT possessed a high hole mobility of 0.12 cm2 V−1 s−1, which was higher than that of the counterpart PDT-DTBT-DT (0.04 cm2 V−1 s−1). Through simplified device optimization without any additives and annealing treatment, a power conversion efficiency (PCE) of 7.86% was achieved for PTVT-DTBT-DT with a short-circuit current density (Jsc) of 16.33 mA cm−2 and a fill factor (FF) of 68.92%, which is higher the PCE of 7.29% of PDT-DTBT-DT with a Jsc of 15.60 mA cm−2 and a FF of 66.62%. The PCE of 7.86% is among the highest PCEs reported for devices fabricated without any additives and thermal annealing treatment. The results revealed that PTVT-DTBT-DT as an ideal conjugated polymer could provide a greater possibility for the commercial application of PSCs, especially in terms of low cost and manufacturing convenience.
Introduction
Bulk heterojunction (BHJ) polymer solar cells (PSCs), an important strategy of utilizing renewable energies, have attracted great attention in the past few decades, due to their great potential application in fabricating low-cost, large-area and flexible photovoltaic devices using printing technologies.1–4 After years of research, using donor–acceptor (D–A) alternating structures to design novel donor materials has been proved to be one of the most effective and successful strategies, because the optical and electrochemical properties of donor materials can be easily tuned by modifying suitable D and A units.5,6 To date, the power conversion efficiency (PCE) of PSCs based on D–A alternating conjugated polymers has been progressively improved to over 11% for single and tandem solar cells.7–21 However, these previously reported works have involved enormous work on device optimization to achieve high PCE, such as thermal annealing treatment, solvent additives, coating cathode interlayer etc., and the device processing complexity and cost disadvantage the commercial application of PSCs in the future. Moreover, as a widely used additive, 1,8-diiodooctane (DIO) has made an indelible contribution to promote the photovoltaic performance of PSCs.22–24 But it can't be ignored that DIO is an undesirable toxic and persistent chemical. As the above discussion, looking for new ideal donor materials with simplified device fabrication processing has been the key for PSCs to be utilized in future commercialization.In order to pursue the high photovoltaic performance of PSCs, appropriate phase separation with suitable domain size between donor and acceptor materials in the active layer is necessary, because it can provide a nanoscale bicontinuous interpenetrating network, which is vital for efficient exciton diffusion, dissociation and electron/hole transportation to the electrode, and this usually results into an enhanced short-circuit current density (Jsc) and higher charge carrier mobility.25,26 The control of morphology with interpenetrating channel-like domains can be achieved by the annealing treatment at elevated temperatures or processing additives with high boiling point, which well explains why so many outstanding works focus on the morphological modulation of active layers by device processing technologies. Sun et al.14 reported a novel conjugated polymer PBDF-T1 with a PCE of 2.64% obtained without solvent additives. While after 1% DIO added, smooth and uniform surface morphology with suitable domain sizes was realized, and consequently a high PCE of 9.43% with higher Jsc was achieved. Woo et al.19 constructed a series of conjugated polymers with good backbone planarity and intermolecular interaction and a PCE of over 7% was achieved without any post-treatment. Then through optimization with diphenyl ether (DPE) and methanol (MeOH) treatment, the Jsc and fill factor (FF) were enhanced to achieve a high PCE of 9.39% eventually. Yan and co-workers7,9,13 did lots of excellent work on side-chain engineering and the device optimization, and they found the annealing treatment and solvent additives could provide ‘optimum PSC morphology’ containing highly crystalline and reasonable polymer domains size, and the Jsc and charge mobility were enhanced drastically. But even so, it's still necessary to realize the bicontinuous interpenetrating network with suitable domain size for high Jsc and hole mobility just by modulating polymer structure with simple or even no device optimization.
As one of the design strategies to improve Jsc and hole mobility, the planarization of polymer structure (even polymer crystallization) can not only improve the degree of π orbitals overlap to extend conjugation length for red-shifted spectrum absorption, but strengthen the interaction between the main chains for stronger π–π stacking.27–29 Hou et al.30 replaced 2-ethylhexyl with octyl to construct PBDTTS1 to improve the ordered intermolecular stacking, and as a consequence, the Jsc was enhanced to 17.46 mA cm−2 with a high PCE of 9.48%. Yang et al.31 changed the backbone conformation of benzo[1,2-b:4,5-b]dithiophene and thieno[3,4-c]pyrrole-4,6-dione (BDT-TPD) based copolymers to a straight one by introducing 3-hexylthieno[3,2-b]-thiophene as the π bridge, which resulted in higher Jsc, higher hole mobility and significantly improved PCE.
(E)-1,2-Di(thiophen-2-yl)ethene (TVT) unit based conjugated polymers have attracted interest in PSCs32–37 and organic field-effect transistors (OFETs),38–41 due to the relatively planar conjugated structure that can extend the conjugation length and provide strong π–π stacking between polymer backbones. In this work, we investigated the effect of vinylene block on the polymer backbone on the hole mobility and photovoltaic properties, and constructed a novel polymer PTVT-DTBT-DT by selecting TVT unit as donor, 5,6-difluorobenzothiadiazole (BT) unit as acceptor and 2-alkylthiophene as π bridge. The previous works have demonstrated that fluorine atom could not only significantly increase the backbone planarity and ordered molecular packing, but improve hole mobility in some cases.42,43 Here, another polymer PDT-DTBT-DT was also synthesized for comparison. PTVT-DTBT-DT exhibited a wide UV-vis absorption covering the while visible region from 400 nm to 800 nm, and the OFETs device based on PTVT-DTBT-DT and PDT-DTBT-DT showed 0.12 cm2 V−1 s−1 and 0.04 cm2 V−1 s−1 mobility respectively. The active layer of PTVT-DTBT-DT/PC71BM achieved appropriate phase separation with more reasonable domain size than that of PDT-DTBT-DT/PC71BM, and consequently a high PCE of 7.86% was achieved by simplified device optimization, which was the highest one among the reported TVT based conjugated polymers32–35 and also among devices fabricated without any additives and post-annealing treatment.44 During the preparation of our article, Cho et al.45 reported the same polymer (named PV4T2FBT) as donor material to investigate a nonfullerene small molecule acceptor (SF-PDI4), and it should be noted that their study focus and device structure were completely different from our work.
Results and discussion
To improve the polymers' solubility, 2-decyltetradecyl alkyl chain was introduced to the backbone to synthesize the target polymer PTVT-DTBT-DT, and the compared polymer PDT-DTBT-DT with the same alkyl chains was also synthesized. As shown in Scheme 1, PTVT-DTBT-DT and PDT-DTBT-DT were polymerized with the corresponding monomers (M1 and M3, M2 and M3) by Stille coupling polymerization reaction using Pd2(dba)3 and P(o-tol)3 as catalyst, anhydrous toluene as solvent. Here, M1, M2 and M3 were synthesized according to previously reported methods.38,46–48 PTVT-DTBT-DT and PDT-DTBT-DT with longer side chains can be readily dissolved in toluene, chlorobenzene, 1,2-dichlorobenzene and hot tetrahydrofuran. Their molecular weight was estimated by gel permeation chromatography (GPC) using THF as solvent and PDT-DTBT-DT shows a number-average molecular weight (Mn) of 349.54 kDa, a weight-average molecular weight (Mw) of 572.41 kDa and a polydispersity index (PDI) of 1.64 and PTVT-DTBT-DT shows a Mn of 317.9 kDa, a Mw of 516.2 kDa and a PDI of 1.62. The thermal stability of these two polymers was investigated by thermo gravimetric analysis (TGA) under a nitrogen atmosphere, and as shown in Fig. 1a, and the onset decomposition temperatures (Td) corresponding to 5% weight loss of PDT-DTBT-DT and PTVT-DTBT-DT are 416.13 °C and 450.24 °C respectively, and the high Td makes sure these two polymers enough thermal stable for PSCs and OFETs application. The crystallinity of PDT-DTBT-DT and PTVT-DTBT-DT films was investigated by the X-ray diffraction (XRD), and the corresponding XRD patterns are shown in Fig. 1b. There are sharp reflection peaks of 100 crystallinity at 2θ = 3.77° and 4.11° for PDT-DTBT-DT and PTVT-DTBT-DT, which corresponding to the distances of 23.42 Å and 21.48 Å between the backbones separated by the side chains respectively, indicating the latter one maybe possess more crystallinity. In addition, PTVT-DTBT-DT has more obvious 200 crystallinity peak at 2θ = 8.07° than that of PDT-DTBT-DT at 2θ = 7.45°.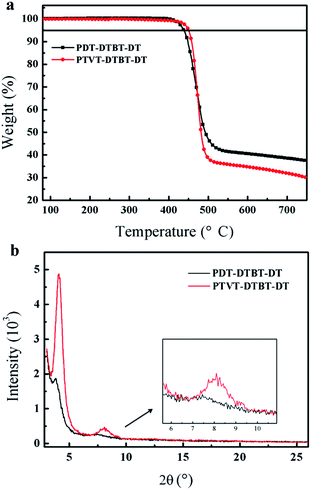 | ||
| Fig. 1 (a) TGA cures of PDT-DTBT-DT and PTVT-DTBT-DT; (b) typical XRD patterns of PDT-DTBT-DT and PTVT-DTBT-DT films. | ||
Optical properties
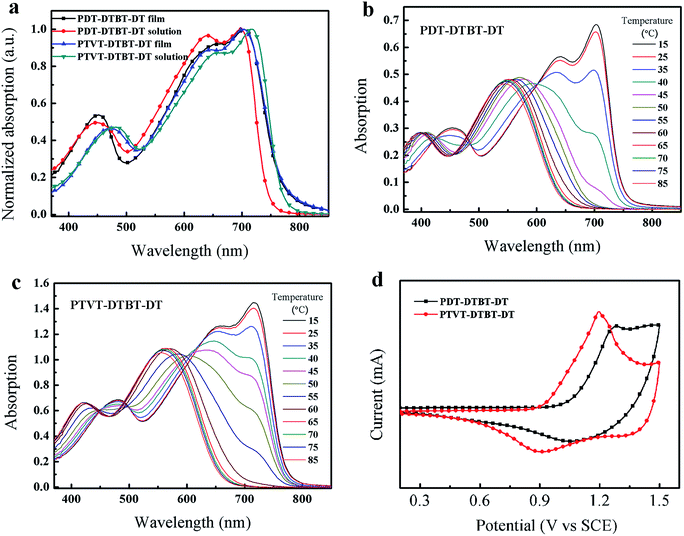
The ultraviolet-visible (UV-vis) absorption spectra of PDT-DTBT-DT and PTVT-DTBT-DT in 1,2-dichlorobenzene solution and in thin solid film are shown in Fig. 2a, and the corresponding absorption properties are summarized in Table 1. Three characteristic absorption peaks can be observed from ultraviolet region to infrared region in solution, and these two short-wavelength peaks (450 nm and 640 nm for PDT-DTBT-DT, 482 nm and 658 nm for PTVT-DTBT-DT) are attributed to the localized π–π* transition of the polymer backbone and the intramolecular charge transfer (ICT) in D–A systems respectively, and the long-wavelength absorption peak (698 nm for PDT-DTBT-DT, 716 nm for PTVT-DTBT-DT) maybe due to the strong intermolecular π–π stacking, which indicates strong aggregation exists between polymer backbones even in the solution state. The film absorption properties of these two polymers are similar with that of solution. But on contrary to the absorption of PDT-DTBT-DT film, the absorption peaks of PTVT-DTBT-DT film is blue-shifted with about 10 nm as opposed to that of solution. The phenomenon rarely happens and it maybe arise from the strong aggregation caused by the enhanced planarity of PTVT-DTBT-DT backbone, where the spin-casting process was fast, and the polymer solution state was suddenly changed, which couldn't achieve the level of aggregation as that in solution. Otherwise, in the solution state, the polymer had enough time to reach the equilibrium state, and the test was carried out in a very dilute solution, which is beneficial to the realization of the thermodynamic equilibrium.49 The absorption onset of PDT-DTBT-DT film is located at 766 nm, corresponding to an optical band gap (Eoptg) 1.62 eV and the Eoptg of PTVT-DTBT-DT is 1.61 eV based on the absorption onset located at 772 nm. It is noteworthy that replacing 2,2′-bithiophene unit (DT) with TVT unit does not change the Eoptg (1.62 eV vs. 1.61 eV).| Polymer | λmax solution (nm) | λon solution (nm) | λmax film (nm) | λon film (nm) | Eoptg (eV) |
|---|---|---|---|---|---|
| PDT-DTBT-DT | 450, 640, 698 | 742 | 452, 654, 704 | 766 | 1.62 |
| PTVT-DTBT-DT | 482, 658, 716 | 768 | 472, 648, 700 | 772 | 1.61 |
To further investigate the intermolecular aggregation interaction of PDT-DTBT-DT and PTVT-DTBT-DT, the evolutive UV-vis absorption spectra of PDT-DTBT-DT and PTVT-DTBT-DT depending on the heating process (from 15 °C to 85 °C) were measured in 1,2-dichlorobenzene solution (1 × 10−5 M), and the corresponding evolutive UV-vis absorption spectra are shown in Fig. 2b and c respectively. With the increase of temperature, the peaks located at 698 nm for PDT-DTBT-DT and 716 nm for PTVT-DTBT-DT are decaying gradually. When the temperature increasing to 60 °C, the peak of PTVT-DTBT-DT disappears, and the temperature is higher 10 °C than that of PDT-DTBT-DT (50 °C). It implies that PTVT-DTBT-DT has stronger intermolecular aggregation than PDT-DTBT-DT. It further illustrates that TVT unit can promote the aggregation between polymer backbone for better absorption.
Electrochemical properties and molecular energy levels
The highest occupied molecular orbital (HOMO) levels of two polymers film were measured by cyclic voltammetry (CV), and the corresponding cyclic voltammogram is shown in Fig. 2d. The onset oxidation potentials (Eox) of PDT-DTBT-DT and PTVT-DTBT-DT are located at 1.02 V and 0.93 V respectively, and according to the equation EHOMO = −e(Eox + 4.4), the corresponding HOMO values were calculated to be −5.42 eV and −5.33 eV respectively. The higher HOMO level of PTVT-DTBT-DT should be ascribed to the improved the inter- and intramolecular π orbital overlap. The lowest unoccupied molecular orbital (LUMO) levels were calculated from the equation ELUMO = EHOMO + Eoptg, and the corresponding values are −3.80 eV and −3.72 eV for PDT-DTBT-DT and PTVT-DTBT-DT respectively. The molecular energy levels are summarized in Table 2.| Polymer | HOMOcv (eV) | LUMO (eV) | Eoptg (eV) | HOMOcal (eV) | LUMOcal (eV) | Ecalg (eV) | θ1 (deg) | θ2 (deg) |
|---|---|---|---|---|---|---|---|---|
| PDT-DTBT-DT | −5.42 | −3.80 | 1.62 | −5.01 | −2.75 | 2.25 | 15.75 | 13.56 |
| PTVT-DTBT-DT | −5.33 | −3.72 | 1.61 | −4.89 | −2.76 | 2.12 | 0.00 | 0.03 |
Theoretical calculations
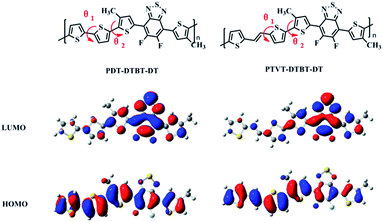
Density functional theory (DFT) calculations were performed by using the Gaussian 09 program at the B3LYP/6-31G (d,p) level in the gas phase to further investigate the molecular backbone conformation of PDT-DTBT-DT and PTVT-DTBT-DT and the corresponding effect on the optoelectronic properties, as our previous work did.50 The dihedral angles, molecular energy levels, band gaps and corresponding electron distributions were calculated and shown in Table 2 and Fig. 3. To avoid excessive computation demand, the alkyl chains attached to backbones in the calculation were replaced by methyl groups. As shown in Fig. 3, θ1 is the inner dihedral angle of donor units (DT unit & TVT unit) and θ2 is the dihedral angle between donor units and thiophene bridges drawn in red. PDT-DTBT-DT has a dihedral angle θ1 of 15.75°, which means an obvious conformational twist between two thiophenes of DT unit. But after vinylene block introduced to donor unit, PTVT-DTBT-DT exhibits an almost planar donor structure (θ1 = 0.00°). Otherwise, PTVT-DTBT-DT has a θ2 of almost 0° (0.03°), which is much smaller than that of PDT-DTBT-DT (θ1 = 13.56°). It maybe attribute to the nearly rigid planar TVT unit efficiently depresses the torsion between adjacent thiophene blocks, and provides better planar conformation of PTVT-DTBT-DT backbone.Charge transport properties
The hole mobilities of PDT-DTBT-DT and PTVT-DTBT-DT were investigated by fabricating OFETs devices. As shown in Fig. 4, typical behaviors of p-type semiconductor can be observed and the corresponding parameters are summarized in Table 3. PTVT-DTBT-DT shows a higher hole mobility of 0.12 cm2 V−1 s−1 with a higher on/off current (Ion/Ioff) ratio of 1.1 × 105 than that of PDT-DTBT-DT with a hole mobility of 0.041 cm2 V−1 s−1 and a Ion/Ioff ratio of 2.7 × 104. The AFM morphology images of PDT-DTBT-DT and PTVT-DTBT-DT films are shown in Fig. S1.† PTVT-DTBT-DT exhibits very smooth morphological structure with a root-mean-square (RMS) of 0.76 nm, which differs from the relative rough morphological structure of PDT-DTBT-DT with a RMS of 1.68 nm. The smooth morphology reveals that PTVT-DTBT-DT possesses better intermolecular aggregation, and hence a higher hole mobility is achieved.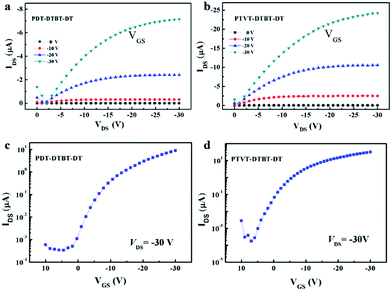 | ||
| Fig. 4 Output (a and b) and transfer characteristics (c and d) of PDT-DTBT-DT and PTVT-DTBT-DT based OFETs. | ||
| Polymer | Ion/Ioff ratio | Threshold voltage (V) | Mobility (cm2 V−1 s−1) |
|---|---|---|---|
| PDT-DTBT-DT | 2.7 × 104 | −4.9 | 0.041 |
| PTVT-DTBT-DT | 1.1 × 105 | −5.0 | 0.12 |
Photovoltaic properties
To investigate the photovoltaic properties of PDT-DTBT-DT and PTVT-DTBT-DT, PSCs devices with a structure of ITO/PEDOT:PSS/polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Ca/Al were fabricated and characterized under the illumination of AM 1.5G, 100 mW cm−2. Here, simple device engineering are employed by adjusting the weight ratio of polymer
PC71BM/Ca/Al were fabricated and characterized under the illumination of AM 1.5G, 100 mW cm−2. Here, simple device engineering are employed by adjusting the weight ratio of polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1.5
PC71BM (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) without any additives and thermal annealing treatment. The current density versus voltage (J–V) cures of devices for PTVT-DTBT-DT and PDT-DTBT-DT are shown in Fig. 5a and Fig. S2,† and the corresponding photovoltaic parameters are shown in Tables 4 and S1.† When the polymer
1.5) without any additives and thermal annealing treatment. The current density versus voltage (J–V) cures of devices for PTVT-DTBT-DT and PDT-DTBT-DT are shown in Fig. 5a and Fig. S2,† and the corresponding photovoltaic parameters are shown in Tables 4 and S1.† When the polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM weight ratio was 1
PC71BM weight ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the devices based on PTVT-DTBT-DT/PC71BM exhibited a high PCE of 7.86% with a Voc of 0.70 V, a Jsc of 16.33 mA cm−2, a FF of 68.92%. When changing the D/A weight ratio to 1
1, the devices based on PTVT-DTBT-DT/PC71BM exhibited a high PCE of 7.86% with a Voc of 0.70 V, a Jsc of 16.33 mA cm−2, a FF of 68.92%. When changing the D/A weight ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 or 1.5
1.5 or 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the PCE slightly decreased with a lower Jsc and a lower FF. To the best of our knowledge, the PCE of 7.86% is the highest one among the reported TVT based conjugated polymers32–37 and is also one of best PSCs fabricated without any additives and post-annealing treatment.44 And moreover, higher PCE with similar molecular structure has been reported recently,7,9,13,15,18,19,21 and their devices were unavoidably fabricated with various additives and post-annealing treatment. Jo and his co-workers reported a series of polymers with different number of fluorine atom, and they achieved a high PCE of 9.14% by adding 1-chloronaphthalene (CN) to the solution with a inverted device structure (ITO/ZnO/PEIE/polymer
1, the PCE slightly decreased with a lower Jsc and a lower FF. To the best of our knowledge, the PCE of 7.86% is the highest one among the reported TVT based conjugated polymers32–37 and is also one of best PSCs fabricated without any additives and post-annealing treatment.44 And moreover, higher PCE with similar molecular structure has been reported recently,7,9,13,15,18,19,21 and their devices were unavoidably fabricated with various additives and post-annealing treatment. Jo and his co-workers reported a series of polymers with different number of fluorine atom, and they achieved a high PCE of 9.14% by adding 1-chloronaphthalene (CN) to the solution with a inverted device structure (ITO/ZnO/PEIE/polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/MoO3/Ag).15 Hou et al. did some work on two highly efficient photovoltaic polymer materials (PDTBT-TT and PBT4T), and they applied the thermal annealing and additives (DIO, CN and DPE) to improve the photovoltaic performance (over 9%).21 But too complex device engineering is not beneficial for the commercialization of PSCs. The devices based on PDT-DTBT-DT/PC71BM at 1
PC71BM/MoO3/Ag).15 Hou et al. did some work on two highly efficient photovoltaic polymer materials (PDTBT-TT and PBT4T), and they applied the thermal annealing and additives (DIO, CN and DPE) to improve the photovoltaic performance (over 9%).21 But too complex device engineering is not beneficial for the commercialization of PSCs. The devices based on PDT-DTBT-DT/PC71BM at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio exhibited an optimized PCE of 7.29% with a Voc of 0.70 V, a Jsc of 15.60 mA cm−2, a FF of 66.62%. Extending π–π conjugation and stronger molecular interaction maybe promote the charge transfer and thus higher Jsc is gotten for PTVT-DTBT-DT. Although PDT-DTBT-DT has deeper HOMO level than PTVT-DTBT-DT, these two polymers exhibit the same Voc. It's because that besides molecular energy levels, other factors such as morphology recombination dynamics and shunt resistance also affect Voc.51–53 The EQE cure (Fig. 5b) of PTVT-DTBT-DT exhibits slightly higher maximum value of 72.9% than 71.2% of PDT-DTBT-DT. Fig. 5c shows the UV-vis absorption spectra of the PTVT-DTBT-DT/PC71BM and PDT-DTBT-DT/PC71BM blend films (1
1 weight ratio exhibited an optimized PCE of 7.29% with a Voc of 0.70 V, a Jsc of 15.60 mA cm−2, a FF of 66.62%. Extending π–π conjugation and stronger molecular interaction maybe promote the charge transfer and thus higher Jsc is gotten for PTVT-DTBT-DT. Although PDT-DTBT-DT has deeper HOMO level than PTVT-DTBT-DT, these two polymers exhibit the same Voc. It's because that besides molecular energy levels, other factors such as morphology recombination dynamics and shunt resistance also affect Voc.51–53 The EQE cure (Fig. 5b) of PTVT-DTBT-DT exhibits slightly higher maximum value of 72.9% than 71.2% of PDT-DTBT-DT. Fig. 5c shows the UV-vis absorption spectra of the PTVT-DTBT-DT/PC71BM and PDT-DTBT-DT/PC71BM blend films (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w), and stronger absorption of PTVT-DTBT-DT/PC71BM are found in the visible region, which well agrees with their EQE curves.
1, w/w), and stronger absorption of PTVT-DTBT-DT/PC71BM are found in the visible region, which well agrees with their EQE curves.
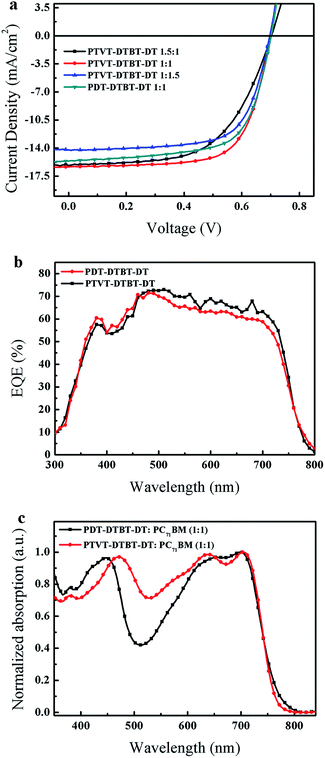 | ||
| Fig. 5 (a) The J–V cures of devices, (b) the EQE cures of devices and (c) normalized UV-vis absorption based on PTVT-DTBT-DT/PC71BM blends and PDT-DTBT-DT/PC71BM blends. | ||
Space-charge-limited current (SCLC) method with a device structure of ITO/PEDOT:PSS/polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Au was applied to provide the calculated hole mobilities 2.61 × 10−4 cm2 V−1 s−1 for PTVT-DTBT-DT and 1.55 × 10−4 cm2 V−1 s−1 for PDT-DTBT-DT. The SCLC results show that PTVT-DTBT-DT has better hole mobilities than PDT-DTBT-DT, which is consistent with the result of OFETs.
PC71BM/Au was applied to provide the calculated hole mobilities 2.61 × 10−4 cm2 V−1 s−1 for PTVT-DTBT-DT and 1.55 × 10−4 cm2 V−1 s−1 for PDT-DTBT-DT. The SCLC results show that PTVT-DTBT-DT has better hole mobilities than PDT-DTBT-DT, which is consistent with the result of OFETs.
The morphological properties of the blend films were investigated by tapping mode atomic force microscopy (AFM) and transmission electron microscopy (TEM) and the images are shown in Fig. 6. The RMS surface roughness of PDT-DTBT-DT and PTVT-DTBT-DT blends are 1.40 nm and 2.07 nm respectively, indicating smooth surface of the active layers. As shown in Fig. 6f, differing from PDT-DTBT-DT blends (Fig. 6c), PTVT-DTBT-DT blends exhibit less polymer and fullerene self-aggregation with more reasonable domain size (decreasing from about 20 nm to about 10 nm), and the bicontinuous interpenetrating network becomes more delicate and compact than that of PDT-DTBT-DT blends, which maybe result from that the improvement of the blended degree of polymer and PC71BM and further provides maximized interface area between PTVT-DTBT-DT and PC71BM for efficient exciton diffusion and dissociation, and more transmission channels for electron/hole to the electrodes.54,55 The difference of morphology can well explain the fact that PTVT-DTBT-DT based devices possesses higher Jsc and hole mobility than that of PDT-DTBT-DT.
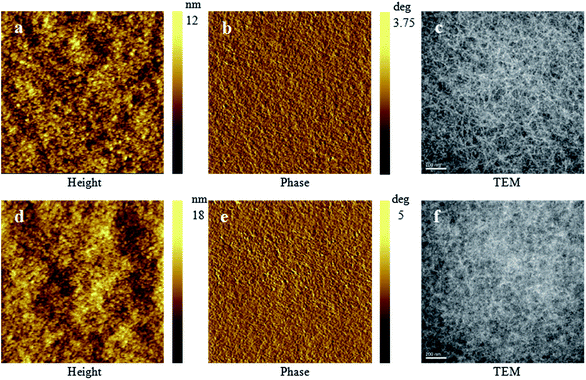 | ||
Fig. 6 AFM (scan size: 4.0 μm × 4.0 μm) and TEM images of PDT-DTBT-DT/PC71BM blend film and PTVT-DTBT-DT/PC71BM blend film (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w): (a–c) for PDT-DTBT-DT, (d–f) for PTVT-DTBT-DT. 1, w/w): (a–c) for PDT-DTBT-DT, (d–f) for PTVT-DTBT-DT. | ||
Conclusions
We synthesized a novel conjugated polymer PTVT-DTBT-DT by selecting TVT unit as donor, BT unit as acceptor and 2-alkylthiophene as π bridge to investigate the effect of polymer backbone conformation on the hole mobility and photovoltaic properties. The planarity of polymer conformation raised by TVT unit can strengthen intermolecular interaction and stronger molecular aggregation was observed. OFETs characterization exhibited a high hole mobility of 0.12 cm2 V−1 s−1, which is higher than that of the compared polymer PDT-DTBT-DT (0.041 cm2 V−1 s−1). Through simplified device optimization without any additives and thermal annealing treatment, a PCE of 7.86% was achieved for PTVT-DTBT-DT, which higher than that of PDT-DTBT-DT with a PCE of 7.29%. The result reveals that PTVT-DTBT-DT as an ideal conjugated polymer can provide a greater possibility for PSCs commercial application, especially in terms of low cost and manufacturing convenience.Experimental
Materials
All of the chemicals are purchased from Energy Chemical, J&K Scientific and other commercial sources. The reagents and solvents were used as received without further purification, except for toluene dried over Na/benzophenone and freshly distilled prior to use. M1, M2, and M3 were synthesized according to the previously reported literature.38,46–48Synthesis of PTVT-DTBT-DT and PDT-DTBT-DT
M1 (103 mg, 0.2 mmol), M2 (233 mg, 0.2 mmol), Pd2(dba)3 (1.86 mg, 0.002 mmol) and P(o-tol)3 (3.6 mg, 0.012 mmol) were dissolved in dry toluene (5 mL). After purged with argon for 30 min, the mixture was heated to 110 °C and stirred for 24 h under argon. Then cooling down to room temperature, the solution was precipitated into MeOH and filtered. The crude product was then purified by Soxhlet extraction with MeOH, hexane and chloroform for 12 hours respectively. Finally, the product PTVT-DTBT-DT was purified by silica gel column chromatography, and dried under vacuum. 198 mg, yield: 82%. 1H NMR (600 MHz, CDCl3): δ (ppm) 8.15–7.87 (br 2H), 7.05–6.52 (br 6H), 2.87–0.78 (m 98H) (Fig. S3†).The synthesis of PDT-DTBT-DT was the same with PTVT-DTBT-DT, 201 mg, yield: 86%. 1H NMR (600 MHz, CDCl3): δ (ppm) 7.99–7.73 (br 2H), 7.06–6.69 (br 4H), 2.73–0.69 (m 98H) (Fig. S4†).
Characterization
1H NMR (nuclear magnetic resonance) spectra were recorded on a Bruker Advance III 600 (600 MHz) with tetramethylsilane (TMS) as an internal standard. The molecular weights of the polymers were measured by GPC using THF as the solvent and polystyrene as the standard under 40 °C. Thermal gravimetric analysis (TGA) measurement was performed by SDT Q600 with a heating rate of 10 °C min−1 under a nitrogen atmosphere. The XRD spectrum was obtained on a Hitachi S-4800. UV-vis absorption spectra were recorded by PerkinElmer Lambda 25 spectrophotometer. Cyclic voltammetry (CV) measurements were performed on a CHI660D electrochemical workstation under an argon atmosphere with a three-electrode cell (a Pt working electrode, a Pt wire counter electrode and an SCE reference electrode) in 0.1 M solution of Bu4NPF6 in acetonitrile at a scan rate of 50 mV s−1 at ambient temperature. DFT calculations were carried out by the Gaussian 09 program suite at the B3LYP/6-31G (d,p) level in the gas phase. The optimized molecular geometries were confirmed to be minimum-energy conformations by computing vibrational frequencies at the same level. Morphological characterizations were characterized by a tapping-mode atomic force microscope (AFM, Agilent 5400) and transmission electron microscope (TEM, HITACHI H-7650).Fabrication and characterization of OFETs devices
Bottom-gate/top-contact OFETs were fabricated on the top of 100 nm thick thermal silicon oxide (SiO2) with heavily n-doped silicon substrates as gate electrodes. After being ultrasonic cleaning by acetone, isopropanol and DI-water for 15 min respectively, the substrates were coated with a self-assembling monolayer of hexamethyldisilazane (HMDS). Then, polymer solution (8 mg mL−1), dissolved in hot 1,2-dichlorobenzene, was spin-coated on SiO2 substrates at a speed of 1200 rpm for 1 min, followed by an post annealing process at 120 °C for 30 minutes on a hot plate in the Mbraun nitrogen glove box. After then, a gold film (100 nm) was deposited on the top of the active layer as source/drain electrodes through a patterned shadow mask (channel length/width = 50 μm/1000 μm) at a rate of 0.2 Å s−1.Fabrication and characterization of PSCs devices
The solar cell devices were fabricated with a structure of ITO/PEDOT:PSS/polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/Ca/Al on 15 mm × 15 mm patterned indium tin oxide (ITO) coated glass substrates. The ITO glass was cleaned in an ultrasonic bath with acetone, methanol and isopropyl alcohol sequentially. After oxygen plasma treatment for 20 min, the substrate was spin-coated with a thin layer of PEDOT:PSS (30 nm) and then dried under argon at 120 °C for 20 min. The active layer was prepared by spin-coating a blend solution of polymer and PC71BM (10 mg mL−1) with different weight ratios (1.5
PC71BM/Ca/Al on 15 mm × 15 mm patterned indium tin oxide (ITO) coated glass substrates. The ITO glass was cleaned in an ultrasonic bath with acetone, methanol and isopropyl alcohol sequentially. After oxygen plasma treatment for 20 min, the substrate was spin-coated with a thin layer of PEDOT:PSS (30 nm) and then dried under argon at 120 °C for 20 min. The active layer was prepared by spin-coating a blend solution of polymer and PC71BM (10 mg mL−1) with different weight ratios (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) in 1,2-dichlorobenzene onto the ITO/PEDOT:PSS electrode. Then, the Ca/Al cathode was deposited on the active layer by vacuum evaporation under 3 × 10−4 Pa. The current density–voltage (J–V) characteristics were measured with a Keithley 2420 source measurement unit under simulated 100 mW cm−2 (AM 1.5G) irradiation from a Newport solar simulator. Light intensity was calibrated with a standard silicon solar cell. The thickness of the active layers was determined by Dektak 150 profilometer, about 100 nm. The EQE of solar cells were analyzed using a certified Newport incident photon conversion efficiency (IPCE) measurement system.
1.5) in 1,2-dichlorobenzene onto the ITO/PEDOT:PSS electrode. Then, the Ca/Al cathode was deposited on the active layer by vacuum evaporation under 3 × 10−4 Pa. The current density–voltage (J–V) characteristics were measured with a Keithley 2420 source measurement unit under simulated 100 mW cm−2 (AM 1.5G) irradiation from a Newport solar simulator. Light intensity was calibrated with a standard silicon solar cell. The thickness of the active layers was determined by Dektak 150 profilometer, about 100 nm. The EQE of solar cells were analyzed using a certified Newport incident photon conversion efficiency (IPCE) measurement system.
Acknowledgements
The authors gratefully acknowledge financial support from the NSFC (21274134, 21274161 and 51573205).References
- L. Huo, S. Zhang, X. Guo, F. Xu, Y. Li and J. Hou, Angew. Chem., Int. Ed., 2011, 50, 9697 CrossRef CAS PubMed.
- L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed.
- S. Liu, K. Zhang, J. Lu, J. Zhang, H. L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2013, 135, 15326 CrossRef CAS PubMed.
- T. Y. Chu, J. Lu, S. Beaupre, Y. Zhang, J. R. Pouliot, S. Wakim, J. Zhou, M. Leclerc, Z. Li, J. Ding and Y. Tao, J. Am. Chem. Soc., 2011, 133, 4250 CrossRef CAS PubMed.
- Z. G. Zhang and J. Wang, J. Mater. Chem., 2012, 22, 4178 RSC.
- Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed.
- H. Hu, K. Jiang, G. Yang, J. Liu, Z. Li, H. Lin, Y. Liu, J. Zhao, J. Zhang, F. Huang, Y. Qu, W. Ma and H. Yan, J. Am. Chem. Soc., 2015, 137, 14149 CrossRef CAS PubMed.
- S. H. Liao, H. J. Jhuo, P. N. Yeh, Y. S. Cheng, Y. L. Li, Y. H. Lee, S. Sharma and S. A. Chen, Sci. Rep., 2014, 4, 6813 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176 CrossRef CAS PubMed.
- S. Zhang, L. Ye, W. Zhao, B. Yang, Q. Wang and J. Hou, Sci. China: Chem., 2015, 58, 248 CrossRef CAS.
- J. Zhao, Y. Li, G. Yang, K. Jiang, H. Lin, H. Ade, W. Ma and H. Yan, Nat. Energy, 2016, 1, 15027 CrossRef.
- L. Huo, T. Liu, B. Fan, Z. Zhao, X. Sun, D. Wei, M. Yu, Y. Liu and Y. Sun, Adv. Mater., 2015, 27, 6969 CrossRef CAS PubMed.
- J. W. Jo, J. W. Jung, E. H. Jung, H. Ahn, T. J. Shin and W. H. Jo, Energy Environ. Sci., 2015, 8, 2427 CAS.
- J. W. Jung, F. Liu, T. P. Russell and W. H. Jo, Adv. Mater., 2015, 27, 7462 CrossRef CAS PubMed.
- J.-H. Kim, J. B. Park, I. H. Jung, A. C. Grimsdale, S. C. Yoon, H. Yang and D.-H. Hwang, Energy Environ. Sci., 2015, 8, 2352 CAS.
- S. H. Liao, H. J. Jhuo, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766 CrossRef CAS PubMed.
- T. L. Nguyen, H. Choi, S. J. Ko, M. A. Uddin, B. Walker, S. Yum, J. E. Jeong, M. H. Yun, T. J. Shin, S. Hwang, J. Y. Kim and H. Y. Woo, Energy Environ. Sci., 2014, 7, 3040 CAS.
- M. Zhang, X. Guo, W. Ma, H. Ade and J. Hou, Adv. Mater., 2015, 27, 4655 CrossRef CAS PubMed.
- S. Zhang, B. Yang, D. Liu, H. Zhang, W. Zhao, Q. Wang, C. He and J. Hou, Macromolecules, 2016, 49, 120 CrossRef CAS.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS PubMed.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS PubMed.
- J. Peet, M. L. Senatore, A. J. Heeger and G. C. Bazan, Adv. Mater., 2009, 21, 1521 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- X. Wang, Y. Sun, S. Chen, X. Guo, M. Zhang, X. Li, Y. Li and H. Wang, Macromolecules, 2012, 45, 1208 CrossRef CAS.
- H. Zhou, L. Yang, A. C. Stuart, S. C. Price, S. Liu and W. You, Angew. Chem., Int. Ed., 2011, 50, 2995 CrossRef CAS PubMed.
- N. Wang, Z. Chen, W. Wei and Z. Jiang, J. Am. Chem. Soc., 2013, 135, 17060 CrossRef CAS PubMed.
- L. Ye, S. Zhang, W. Zhao, H. Yao and J. Hou, Chem. Mater., 2014, 26, 3603 CrossRef CAS.
- S. Liu, X. Bao, W. Li, K. Wu, G. Xie, R. Yang and C. Yang, Macromolecules, 2015, 48, 2948 CrossRef CAS.
- J. W. Jung, T. P. Russell and W. H. Jo, ACS Appl. Mater. Interfaces, 2015, 7, 13666 CAS.
- H. Chen, Y. Guo, Z. Mao, G. Yu, J. Huang, Y. Zhao and Y. Liu, Chem. Mater., 2013, 25, 3589 CrossRef CAS.
- J. Choi, K.-H. Kim, H. Yu, C. Lee, H. Kang, I. Song, Y. Kim, J. H. Oh and B. J. Kim, Chem. Mater., 2015, 27, 5230 CrossRef CAS.
- A. Cardone, C. Martinelli, M. Losurdo, E. Dilonardo, G. Bruno, G. Scavia, S. Destri, P. Cosma, L. Salamandra, A. Reale, A. Di Carlo, A. Aguirre, B. Milián-Medina, J. Gierschner and G. M. Farinola, J. Mater. Chem. A, 2013, 1, 715 CAS.
- F. Qing, Y. Sun, X. Wang, N. Li, Y. Li, X. Li and H. Wang, Polym. Chem., 2011, 2, 2102 RSC.
- Y. R. Cheon, Y. J. Kim, J.-j. Ha, M.-J. Kim, C. E. Park and Y.-H. Kim, Macromolecules, 2014, 47, 8570 CrossRef CAS.
- H. Chen, Y. Guo, G. Yu, Y. Zhao, J. Zhang, D. Gao, H. Liu and Y. Liu, Adv. Mater., 2012, 24, 4618 CrossRef CAS PubMed.
- H. Huang, Z. Chen, R. Ponce Ortiz, C. Newman, H. Usta, S. Lou, J. Youn, Y. Y. Noh, K. J. Baeg, L. X. Chen, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2012, 134, 10966 CrossRef CAS PubMed.
- R. Kim, P. S. K. Amegadze, I. Kang, H.-J. Yun, Y.-Y. Noh, S.-K. Kwon and Y.-H. Kim, Adv. Funct. Mater., 2013, 23, 5719 CrossRef CAS.
- I. Kang, H. J. Yun, D. S. Chung, S. K. Kwon and Y. H. Kim, J. Am. Chem. Soc., 2013, 135, 14896 CrossRef CAS PubMed.
- Z. Li, J. Lu, S.-C. Tse, J. Zhou, X. Du, Y. Tao and J. Ding, J. Mater. Chem., 2011, 21, 3226 RSC.
- H. Bronstein, J. M. Frost, A. Hadipour, Y. Kim, C. B. Nielsen, R. S. Ashraf, B. P. Rand, S. Watkins and I. McCulloch, Chem. Mater., 2013, 25, 277 CrossRef CAS.
- G. Li, X. Gong, J. Zhang, Y. Liu, S. Feng, C. Li and Z. Bo, ACS Appl. Mater. Interfaces, 2016, 8, 3686 CAS.
- J. Lee, R. Singh, D. H. Sin, H. G. Kim, K. C. Song and K. Cho, Adv. Mater., 2016, 28, 69 CrossRef CAS PubMed.
- M. Pei, J. Huang, M. Jang, J.-H. Kim, M. Lee, J. Chen, D. H. Hwang and H. Yang, J. Phys. Chem. C, 2016, 120, 903 CAS.
- Z. Chen, P. Cai, J. Chen, X. Liu, L. Zhang, L. Lan, J. Peng, Y. Ma and Y. Cao, Adv. Mater., 2014, 26, 2586 CrossRef CAS PubMed.
- Q. Wu, S. Ren, M. Wang, X. Qiao, H. Li, X. Gao, X. Yang and D. Zhu, Adv. Funct. Mater., 2013, 23, 2277 CrossRef CAS.
- J. Zhao, Y. Li, A. Hunt, J. Zhang, H. Yao, Z. Li, J. Zhang, F. Huang, H. Ade and H. Yan, Adv. Mater., 2016, 28, 1868 CrossRef CAS PubMed.
- J. Ren, X. Bao, L. Han, J. Wang, M. Qiu, Q. Zhu, T. Hu, R. Sheng, M. Sun and R. Yang, Polym. Chem., 2015, 6, 4415 RSC.
- M. D. Perez, C. Borek, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2009, 131, 9281 CrossRef CAS PubMed.
- W. J. Potscavage, A. Sharma and B. Kippelen, Acc. Chem. Res., 2009, 42, 1758 CrossRef CAS PubMed.
- A. Maurano, R. Hamilton, C. G. Shuttle, A. M. Ballantyne, J. Nelson, B. O'Regan, W. Zhang, I. McCulloch, H. Azimi, M. Morana, C. J. Brabec and J. R. Durrant, Adv. Mater., 2010, 22, 4987 CrossRef CAS PubMed.
- W. Li, K. H. Hendriks, A. Furlan, W. Roelofs, S. C. Meskers, M. M. Wienk and R. A. Janssen, Adv. Mater., 2014, 26, 1565 CrossRef CAS PubMed.
- W. Li, K. H. Hendriks, A. Furlan, W. Roelofs, M. M. Wienk and R. A. Janssen, J. Am. Chem. Soc., 2013, 135, 18942 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13970a |
| ‡ J. Ren and Y. Zhang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |