A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method
Fang Wanga,
Hairong Li*abd,
Zhaoxin Yuana,
Yongzhe Suna,
Fangzhi Changa,
Heng Denga,
Longzhen Xiea and
Haiyan Lic
aSchool of Physical Science and Technology, Lanzhou University, South Tianshui Road 222#, Lanzhou 730000, China. E-mail: hrli@lzu.edu.cn; Tel: +86 15002590498
bKey Laboratory for Magnetism and Magnetic Materials of the Ministry of Education, Lanzhou University, South Tianshui Road 222#, Lanzhou 730000, China
cSchool of Life Science, Lanzhou University, South Tianshui Road 222#, Lanzhou, China
dInstitute of Sensor Technology, Gansu Academy of Sciences, China
First published on 3rd August 2016
Abstract
In this paper, CuO nanoparticles were synthetized via a sol–gel method and their corresponding gas sensor was achieved simultaneously. CuO nanoparticle samples were characterized by X-ray diffraction, Raman spectroscopy, X-ray photoelectron spectroscopy and field emission scanning electron microscopy, respectively. The results show that the sample we have synthesized was CuO and the morphology of the sample was nanoparticles with an average diameter of ∼100 nm. We determined the operating temperature of the gas sensors to be 220 °C, considering their appropriate sensitivity, rapid response and the uniformity of testing. Under this working temperature, the sensitivity and response/recovery time of the gas sensor were tested with acetone, methanol and ethanol gas. It was found that the CuO nanoparticles gas sensor performed a high response to the low concentrations of these three gases. At a gas concentration of 0.1 ppm and 10 ppm, the response to the three gases was about 2.5, 1.9, 2.7 and 5.3, 5.9, 5.3, respectively. It is believed that the CuO nanoparticles may be a promising candidate for low concentration reducing volatile organic gases sensing applications.
I. Introduction
Air pollution problems are drawing more and more people's attention with the development of modern industry. Gas sensors that can detect a variety of volatile organic gases (VOCs) or toxic gases are of great importance. Currently, researchers have developed a various kinds of gas sensor, such as oxide semiconductors,1 organic semiconductors,2 field effect types3 and surface acoustic wave types.4 Wherein, the oxide semiconductor gas sensor have attracted people's attention because of its high sensitivity, low manufacturing cost and simple means of measurement and signal characteristics. However, most commercial products still use sensing materials based on SnO2, WO3 and ZnO, which are n-type semiconductors, for VOCs or toxic gas detection.5–14 When compared to the n-type metal oxide gas sensor, the p-type metal oxide gas sensor has not only a few shortcomings, but also has great potential value in practical applications, especially in reducing volatile organic gases (VOCs). Hübner et al.15 suggested that the response of a p-type oxide semiconductor gas sensor to a given gas was equal to the square root of that found for an n-type oxide semiconductor gas sensor to the same gas when the morphological configurations of both sensor materials were identical. For an n-type oxide semiconductor, it will form an electron-depletion layer (EDL), but for an p-type oxide semiconductor, it will form a hole-accumulation layer (HAL) by the adsorption of oxygen with negative charge. When exposed to reducing VOCs, the bulk resistance of an n-type semiconductor oxide gas sensor is reduced, while the p-type semiconductor oxide gas sensor bulk resistance becomes larger. Therefore, it is very important to study the p-type oxide semiconductor gas sensing device. However, research on the p-type oxide semiconductor gas sensor is rare.Cupric oxide (CuO) is one of the most important p-type oxide semiconductors as it exhibits a stable narrow band gap (1.2–1.9 eV).16 Nano CuO and its composite oxides have potential applications in the field of sensor because of their large surface-area-to-volume ratio. Various nanostructured CuO materials, for example, nanowires,17 nanorods,18–20 nanotubes,21 nanoflowers22 and nanoparticles23,24 have been synthesized and investigated for their gas sensing properties. However, CuO is mainly used as a kind of p-type dopant or catalyst and introduced into a typical n-type semiconductor, such as SnO2 (ref. 25) or ZnO,26 which is very small in the material composition. It is rarely reported that pure CuO materials have been used as sensors. In the existing CuO based gas sensors, CuO micro/nanostructures based on different sizes and morphologies have been reported. For example, Chao Yang et al.27 have used CuO nanoparticles as gas sensitive materials prepared via a chemical precipitation method and the optimal sensitivity was about 2.0 at a concentration of 10 ppm ethanol gas. Generally speaking, the gas sensor, which is separated from the sensitive body and the heater, is called an indirectly heated gas sensor. Most indirectly heated gas sensors process the sensitive material into a powder and coat it onto the ceramic tube. However, the indirectly heated p-type nano CuO gas sensor process is complex. When compared with their preparation processes, CuO gas sensitive materials are directly grown on the Al2O3 ceramic tube with Au electrodes via sol–gel and annealing methods, which leads to a reduction in the complexity of the sensor process. Otherwise, the detection concentration of acetone, methanol, ethanol and other reducing gases is tens or even hundreds and thousands more than ppm, and the p-type CuO gas sensor exhibits a high bulk resistance and working temperature.
In this paper, an indirectly heated gas sensor based on p-type nanoparticles CuO was fabricated via sol–gel and thermal annealing methods. The gas sensor adopts the p-type CuO as the main material without doping, which is not only friendly to the environment, but also an innovation in the processes that promote efficiency and cost savings. The fabricated CuO nanoparticles sensor exhibits excellent gas sensing behavior towards acetone, methanol and ethanol. Its working temperature was relatively low (200–250 °C) and when compared with other metal oxide based sensors, the sensor based on CuO nanoparticles we have fabricated via a sol–gel method can detect a low concentration (0.1 ppm) to acetone, methanol and ethanol, and the response of these gases was about 2.5, 1.9, 2.7, respectively. For example, Peige Zhang et al.28 have synthesized SnO2 spheres, corals, sheets and octahedrons via hydrothermal routes with tin salts under different conditions. They have tested the gas response under an ethanol concentration of 50 ppm at 340 °C, corresponding to a sensitivity of about 5.
II. Experimental and procedures
A. Synthesis of CuO nanoparticles
All the chemical reagents used in this research were analytical grade and used directly without further purification. The main materials used in this experiment are copper acetate (AR, General Reagent Factory, Shanghai), iso-propyl alcohol (AR, Kelong Chemical Reagent Factory, Chengdu), ethanolamine (AR, Chengdu Gray Chemical Technology Co., Ltd.), methanol (AR, ≥99.7%), ethanol (AR, ≥99.7%) and acetone (AR, ≥99.7%) (Tianjin KaiXin Chemical Co. Ltd.). In a typical procedure, 1.0 g of Cu(CH3COO)2·H2O was dissolved into 15 mL of iso-propyl alcohol. Meanwhile, 1 mL of the stabilizer ethanolamine was added to the reaction mixture. Afterwards, the mixed solution was heated at 80 °C under constant magnetic stirring for 2 h. After that, when the reaction was complete, the sol–gel was allowed to cool to room temperature naturally. Eventually, the final dark-blue mixture was allowed to stand at room temperature for 24 h. The primary product obtained from this process was used to prepare the nanoparticles gas sensing devices.B. Fabrication of the gas sensing devices
The indirectly heated gas sensor was fabricated and the configuration of the device is shown in Fig. 1(a). 5 μL of the as-prepared sol–gel liquid was dropped on a horizontal Al2O3 ceramic tube wall and the tube rotated horizontally at a constant velocity in order to form a uniform film, until the liquid completely covered ceramic tube. After drying in an oven, the ceramic tube with a sol–gel solution layer was annealed at 500 °C for 2 h in air conditions. The chemical reaction of the overall synthetic process is assumed to be as follows:| Cu(CH3COO)2·H2O → CuO↓ + CH4↑ + CO2↑ |
 | ||
| Fig. 1 (a) The cross-section drawn of the CuO nanoparticles gas sensor. (b) A schematic diagram of the gas sensor testing system. | ||
Cupric acetate can be decomposed into CuO, CH4 and CO2 when heated in air. After that, CuO was annealed at 500 °C for 2 h in air conditions for crystallization and phase formation. Finally, four pin electrodes of the ceramic tube and the Ni–Cr alloy wire were welded on the pedestal of the ceramic tube.
C. Characterization
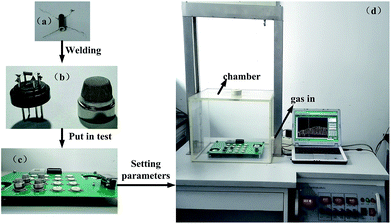
The sensing properties of the sensor were measured using a QJC-II series Intelligent Test Meter. A schematic diagram of the gas sensor testing system is shown in Fig. 1(b). The specific testing process is shown in Fig. 2. The relative humidity was regulated in the range of 30–40% by dehumidification using an air conditioner. To calculate the p-type character of the CuO nanoparticles gas sensor, the sensitivity (response) S was defined as:| S = Rgas/Rair | (1) |
The as-synthesized CuO nanoparticles were characterized by X-ray diffraction (XRD, Rigaku D/max-2400 with Cu Kα radiation), micro-Raman spectroscopy (Jobin-Yvon Horiba HR800 with an excitation wavelength of 532 nm), X-ray photoelectron spectroscopy (Kratos Axis UltraDLD with a monochrome Al target and double anode Al/Mg target). The morphology of the products was examined using field emission scanning electron microscopy (SEM, Hitachi S-4800).
III. Results and discussion
A. Characterization of the CuO nanoparticles
The phase structure of the obtained precursor was determined by XRD. Fig. 3(a) shows the X-ray diffraction (XRD) pattern of the CuO nanoparticles after being annealed at 500 °C for 2 h. The samples were scanned from 20° to 100° with an increment of 0.02 at a scan speed of 0.2 s per step. The peaks of the as-synthesized CuO nanomaterial at 2θ = 32.48°, 35.76°, 38.92°, 49°, 61.8°, 66.46° and 68.26° were indexed to the (110) (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11), (111), (
11), (111), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 13), (
13), (![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 11) and (220) planes, respectively. This confirms that the obtained samples are crystalline and have the monoclinic structure of CuO (JCPDS: 80-0076). Fig. 3(b) presents the Raman spectra of the CuO nanoparticles samples, annealed in air at 500 °C for 2 h. It can be seen that there are four Raman peaks at 297, 346, 631 and 1105 cm−1. Fig. 3(c) and (d) shows the corresponding XPS spectra of the CuO nanoparticles. As depicted in Fig. 3(c), the Cu 2p3/2 and Cu 2p1/2 peaks of the CuO nanoparticles are centered at 933.5 eV and 953.4 eV, respectively. The gap between the Cu 2p3/2 and Cu 2p1/2 peaks was 19.9 eV, which is consistent with standard spectrum of CuO. The O 1s peak of the sample is shown in Fig. 3(d) and can be fitted into two components using a Gaussian function. The two components are centered at 529.7 eV and 532.1 eV, corresponding to Cu–O of CuO and oxygen absorbed on the surface CuO, respectively. These results indicate that the sample was composed of CuO.
11) and (220) planes, respectively. This confirms that the obtained samples are crystalline and have the monoclinic structure of CuO (JCPDS: 80-0076). Fig. 3(b) presents the Raman spectra of the CuO nanoparticles samples, annealed in air at 500 °C for 2 h. It can be seen that there are four Raman peaks at 297, 346, 631 and 1105 cm−1. Fig. 3(c) and (d) shows the corresponding XPS spectra of the CuO nanoparticles. As depicted in Fig. 3(c), the Cu 2p3/2 and Cu 2p1/2 peaks of the CuO nanoparticles are centered at 933.5 eV and 953.4 eV, respectively. The gap between the Cu 2p3/2 and Cu 2p1/2 peaks was 19.9 eV, which is consistent with standard spectrum of CuO. The O 1s peak of the sample is shown in Fig. 3(d) and can be fitted into two components using a Gaussian function. The two components are centered at 529.7 eV and 532.1 eV, corresponding to Cu–O of CuO and oxygen absorbed on the surface CuO, respectively. These results indicate that the sample was composed of CuO.
The morphology of the as-synthesized CuO was investigated by SEM, as shown in Fig. 4. Fig. 4(a) and (b) are the SEM images, at relatively low magnification (1.5k and 30k), of the nanoparticles CuO material obtained via a sol–gel process and annealing at 500 °C for 2 h. Fig. 3(c) and (d) are the SEM images at higher magnification (60k and 120k). SEM observations were performed to investigate the morphology of the as-prepared samples. From Fig. 4(a), we can see that the surface of the sample looks wrinkled under low magnification. The wrinkles may result from the large contract area with air. In Fig. 4(b) and (c), it can be clearly seen that there are micropores between the particles, thereby providing more contact area for the gas with the interior materials. Fig. 4(d) shows that the sample is comprised of particles and the size of the particles are ∼100 nm under high magnification.
 | ||
| Fig. 4 (a–d) The SEM photomicrographs of the CuO nanoparticles after being annealed at 500 °C for 2 h at different magnification. | ||
B. Gas sensing performance
After the gas sensor was preheated for half an hour, the gas sensing properties were tested. It is acknowledged that the response of a gas sensor is usually dependent on the temperature. Herein, the sensor based on the CuO nanoparticles was exposed to acetone, methanol and ethanol at a concentration of 1 ppm at different operating temperatures. Fig. 5(a) shows the responses of the CuO nanoparticles sensor to 1 ppm of acetone, methanol and ethanol at different operating temperatures from 180 °C to 290 °C. The temperature dependence of the semiconductor metal oxide based sensor, which has already been reported in the literature,29–32 could be ascribed to the competition between the Knudsen diffusion and surface reaction, which leads to the resistance change of the sensor.33 For acetone, ethanol and methanol gas, the sensor response decreased when the temperature was increased to 220 °C. This is also obviously seen in Fig. 5(a). Considering the power consumption and unity of testing, we analyzed that the operating temperature of three gases for acetone, methanol and ethanol was 220 °C. The gas sensing performance of the as-fabricated sensor was measured at different concentrations of acetone, methanol and ethanol at an appropriate operating temperature and the relative humidity was regulated in the range of 30–40%. Subsequently, the response/recovery time of the three gases was tested, as shown in Fig. 5(b–d), respectively, for a concentration of 1 ppm at the optimum operating temperature. Based on the analysis of the test results, it can be concluded that the response/recovery time to the concentration of 1 ppm acetone, methanol and ethanol was 12 s/8 s, 13 s/13 s and 13 s/9 s, respectively. When compared with the literature, the sensor based on CuO nanoparticles has an advantage in the response/recovery time. For example, Yu Cao et al.34 have synthesized CuO nanoleaves by adjusting the addition of sodium hydroxide and hydrazine hydrate in aqueous solution, and the responses/recover time was about 23–55 s and 20–57 s when the concentration ranged from 50 to 1500 ppm at 260 °C, respectively.Furthermore, the corresponding responses versus the concentration of acetone, methanol and ethanol from 0.1 ppm to 10 ppm (0.1 ppm, 0.2 ppm, 0.4 ppm, 0.5 ppm, 1 ppm, 2 ppm, 5 ppm and 10 ppm) were tested at their appropriate operating temperature and the relative humidity was regulated in the range of 30–40%. The initial resistance of the sensor changed with the target gas injected into the testing chamber. The resistance curves sensor sensitivities of the CuO nanoparticles sensors versus different concentrations of acetone, methanol and ethanol from 0.1 to 10 ppm are displayed in Fig. 6. It can be seen that the CuO nanoparticles show a good response to acetone, methanol and ethanol. Fuchao Yang et al.35 have synthesized a 1D needle-like CuO gas sensor utilizing simple solution-treatment, heat-treatment and sonication processes based on copper meshes (CMs); for 50 ppm acetone, the response value of the needle-like CuO sensor was 3.82 and for 100 ppm acetone, the response value of the needle-like CuO sensor was 4.64. However, the CuO nanoparticles we have synthetized via a sol–gel method display a relatively high sensitivity to low concentration acetone, methanol and ethanol. From the results, it can be found that the response (Rg/Ra) of the CuO nanoparticles gas sensor to acetone gas concentrations at 0.1 and 10 ppm was 2.5 and 5.3, respectively and the response/recovery time are 10 s/6 s, as shown in Fig. 6(a) and (b), respectively. For methanol gas, as shown in Fig. 6(c) and (d), the response (Rg/Ra) was 1.9 and 5.9, respectively. The response/recovery time was 11 s and 6 s respectively. From the Fig. 6(e) and (f), the sensitivity (Rg/Ra) of the ethanol gas was 2.7 and 5.1, respectively and the response/recovery time was 11 s/7 s, respectively. When compared with other sensors based on pure CuO used in the present study, the properties of the sensor's response to 10 ppm of acetone, methanol and ethanol were superior to them. Huiying Yan et al.36 have synthesized CuO nanosheets using a facile solution method combined with subsequent calcination, whose response falls short of 2.0 to 10 ppm acetone, methanol and ethanol under an optimum working temperature of 320 °C and the response/recovery time of the CuO nanosheets-based sensor were 8 s and 9 s towards 100 ppm ethanol gas, respectively. Furthermore, Chao Yang et al.37 have synthetized CuO nanosheets using a microwave-assisted process, whose response to 10 ppm ethanol and acetone was about 1.5 under a working temperature of 260 °C.
Herein, the responses of the CuO nanoparticles gas sensor towards other gases that exist usually in indoor ambient air has also been examined and summarized in Fig. 7. Fig. 7 shows the gas response to 1 ppm acetone, methanol, ethanol, toluene, ammonia and formaldehyde. It can be seen that, among the tested gases, the CuO nanoparticles prepared via the sol–gel method has quite a strong response to ketone and alcohol gases with the most potential to be used as a gas-sensing material in an air quality measurement system (AQMS).
 | ||
| Fig. 7 The response values of the CuO nanoparticles gas sensor to C3H6O, C2H6O, CH4O, C7H8, NH3 and CH2O at a concentration of 1 ppm, respectively under a working temperature of 220 °C. | ||
Fig. 8 shows the gas sensor's responses at different ethanol concentrations at various relative humidity concentrations. The response of the CuO gas sensor was approximately 1.8 at 0.5 ppm ethanol even at a relative humidity of 80%, which indicates that it is relatively stable under a humid atmosphere. The long-term stability is very important for the practical use of a sensor. Commercially available gas sensors require calibration times from 48 h up to 168 h. The author measured the resistance changes with time under specific conditions: the relative humidity was about 30–40% and the sensors were heated up to 220 °C. Fig. 9 shows the baseline resistance changes with time for the CuO nanoparticles gas sensor. The experiment was carried out for 168 h.
 | ||
| Fig. 8 The response of the CuO gas sensor to 0.1–0.5 ppm ethanol measured at different relative humidity. | ||
C. Conduction and gas-sensing mechanism
Oxygen molecules adsorb onto the surface of p-type oxide semiconductors and ionize into species such as O2−, O− and O2− by taking electrons near the surface of the semiconductors under 100–500 °C.38,39 In general, the ionosorption species of O2−, O− and O2− are known to be dominant at <150 °C, between 150 and 400 °C, and at >400 °C, respectively.39 When the CuO gas sensor was exposed to air, the reactions that will occur are:14| O2ads + e− ↔ O2ads− | (2) |
| O2ads− + e− ↔ 2Oads− | (3) |
| Oads− + e− ↔ Oads2− | (4) |
Wherein, the chemical reaction formula on the surface of the CuO nanoparticles to ketones and alcohols such as acetone, methanol and ethanol gas are expressed as follows:
| R–OH + Oads− → CO2 + H2O + e | (5) |
| R–CO–R′ + Oads− → CO2 + H2O + e | (6) |
IV. Conclusions
In summary, a non-doped CuO nanoparticles gas sensor with high response has been successfully fabricated via sol–gel and thermal annealing treatments. The response of the gas sensor to reducing gas at different temperatures and different concentrations was tested. The optimal operating temperature was in the range of 200–250 °C. This temperature range was lower than the average working temperature of previously reported CuO nanomaterial gas sensors. The sensitivity of the device was high at a concentration of 0.1 ppm for acetone, methanol and ethanol gas, its sensitivities were 2.5, 1.9 and 2.7, respectively. The CuO nanoparticles gas sensor could have a very promising application in indoor and drunken driving tests due to its high sensitivity and its production process is simple and easy to mass production.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61204106), Provincial Natural Science Foundation of Gansu (No. 1107RJZA090), Provincial Natural Science Foundation of Gansu (2016GS08252) and Scientific and Technological Project of Chengguan District of Lanzhou (2016cgkj280).Notes and references
- F. Liao, C. Chen and V. Subramanian, Sens. Actuators, B, 2005, 107, 849–855 CrossRef CAS
.
- J. J. Miasik, A. Hooper and B. C. Tofield, J. Chem. Soc., Faraday Trans. 1, 1986, 82, 1117–1126 RSC
.
- N. Peng, Q. Zhang, Y. C. Lee, O. K. Tan and N. Marzari, Sens. Actuators, B, 2008, 132, 191–195 CrossRef CAS
.
- M. Penza, P. Aversa, G. Cassano, W. Wlodarski and K. Kalantarzadeh, Sens. Actuators, B, 2007, 127, 168–178 CrossRef CAS
.
- N. Yamazoe, Sens. Actuators, B, 2005, 108, 2–14 CrossRef CAS
.
- N. Yamazoe, G. Sakai and K. Shimanoe, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS
.
- P. Gao, Y. Chen, Y. Wang, Q. Zhang, X. Li and M. Hu, Chem. Commun., 2009, 2762–2764 RSC
.
- X. L. Cheng, H. Zhao, L. H. Huo, S. Gao and J. G. Zhao, Sens. Actuators, B, 2004, 102, 248–252 CrossRef CAS
.
- Y. Chen, C. L. Zhu and G. Xiao, Nanotechnology, 2006, 17, 4537–4541 CrossRef CAS PubMed
.
- D. Bao, P. Gao, L. Wang, Y. Wang, Y. Chen, G. Chen, G. Li, C. Chang and W. Qin, ChemPlusChem, 2013, 78, 1266–1272 CrossRef CAS
.
- X. Jiaqiang, C. Yuping, L. Yadong and S. Jianian, J. Mater. Sci., 2005, 40, 2919–2921 CrossRef
.
- D.-S. Lee, J.-K. Jung, J.-W. Lim, J.-S. Huh and D.-D. Lee, Sens. Actuators, B, 2001, 77, 228–236 CrossRef CAS
.
- J.-H. Lee, Sens. Actuators, B, 2009, 140, 319–336 CrossRef CAS
.
- H.-J. Kim and J.-H. Lee, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS
.
- M. Hübner, C. E. Simion, A. Tomescu-Stănoiu, S. Pokhrel, N. Bârsan and U. Weimar, Sens. Actuators, B, 2011, 153, 347–353 CrossRef
.
- D. Wu, Q. Zhang and M. Tao, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 235206 CrossRef
.
- Y. H. Ko, G. Nagaraju, S. H. Lee and J. S. Yu, Mater. Lett., 2014, 117, 217–220 CrossRef CAS
.
- H. Kim, C. Jin, S. Park, S. Kim and C. Lee, Sens. Actuators, B, 2012, 161, 594–599 CrossRef CAS
.
- C. Yang, X. Su, F. Xiao, J. Jian and J. Wang, Sens. Actuators, B, 2011, 158, 299–303 CrossRef CAS
.
- C. Wang, X. Q. Fu, X. Y. Xue, Y. G. Wang and T. H. Wang, Nanotechnology, 2007, 18, 145506 CrossRef
.
- Y. Sun, L. Ma, B. Zhou and P. Gao, Int. J. Hydrogen Energy, 2012, 37, 2336–2343 CrossRef CAS
.
- K. Khun, Z. H. Ibupoto and M. Willander, Electroanalysis, 2013, 25, 1425–1432 CrossRef CAS
.
- A. Das, B. Venkataramana, D. Partheephan, A. K. Prasad, S. Dhara and A. K. Tyagi, Phys. E, 2013, 54, 40–44 CrossRef CAS
.
- A. Taubert, F. Stange, Z. Li, M. Junginger, C. Günter, M. Neumann and A. Friedrich, ACS Appl. Mater. Interfaces, 2012, 4, 791–795 CAS
.
- L. He, Y. Jia, F. Meng, M. Li and J. Liu, Asian J. Mater. Sci., 2009, 44, 4326–4333 CrossRef CAS
.
- H. Gong, J. Hu, J. Wang, C. Ong and F. Zhu, Sens. Actuators, B, 2006, 115, 247–251 CrossRef CAS
.
- C. Yang, F. Xiao, J. Wang and X. Su, J. Colloid Interface Sci., 2014, 435, 34–42 CrossRef CAS PubMed
.
- P. Zhang, W. Zeng and L. Chen, J. Mater. Sci.: Mater. Electron., 2016, 27, 1201–1208 CrossRef CAS
.
- C. W. Zou, J. Wang, F. Liang, W. Xie, L. X. Shao and D. J. Fu, Curr. Appl. Phys., 2012, 12, 1349–1354 CrossRef
.
- N. D. Hoa, N. Van Quy, H. Jung, D. Kim, H. Kim and S.-K. Hong, Sens. Actuators, B, 2010, 146, 266–272 CrossRef
.
- A. Aslani and V. Oroojpour, Phys. B, 2011, 406, 144–149 CrossRef CAS
.
- H. Che, A. Liu, J. Hou, J. Mu, Y. Bai, S. Zhao, X. Zhang and H. He, J. Mater. Sci.: Mater. Electron., 2014, 25, 3209–3218 CrossRef CAS
.
- Y. Cao, S. Y. Liu, X. Jian, G. L. Zhu, L. J. Yin, L. Zhang, B. Wu, Y. F. Wei, T. Chen, Y. Q. Gao, H. Tang, C. Wang, W. D. He and W. L. Zhang, RSC Adv., 2015, 5, 34788–34794 RSC
.
- Y. Cao, S. Liu, X. Jian, G. Zhu, L. Yin, L. Zhang, B. Wu, Y. Wei, T. Chen, Y. gao, H. Tang, C. Wang, W. He and W. Zhang, RSC Adv., 2015, 5, 34788–34794 RSC
.
- F. C. Yang, J. Guo, M. M. Liu, S. Yu, N. B. Yan, J. Li and Z. G. Guo, J. Mater. Chem. A, 2015, 3, 20477–20481 CAS
.
- H. Yan, X. Tian, J. Sun and F. Ma, J. Mater. Sci.: Mater. Electron., 2014, 26, 280–287 CrossRef
.
- C. Yang, F. Xiao, J. Wang and X. Su, Sens. Actuators, B, 2015, 207, 177–185 CrossRef CAS
.
- C. O. Park and S. Akbar, J. Mater. Sci., 2003, 38, 4611–4637 CrossRef CAS
.
- N. Barsan and U. Weimar, J. Electroceram., 2001, 7, 143–167 CrossRef CAS
.
- N. Barsan, C. Simion, T. Heine, S. Pokhrel and U. Weimar, J. Electroceram., 2010, 25, 11–19 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |