Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase†
Jian Yang,
Zhe Wang,
Yongsheng Li,
Qixin Zhuang,
Wenru Zhao and
Jinlou Gu*
Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: jinlougu@ecust.edu.cn; Fax: +86-21-64250740; Tel: +86-21-64252599
First published on 12th July 2016
Abstract
Developing an effective method that could provide simple, rapid and visual determination of Hg2+ in water has attracted great attention. In this work, a novel chemical sensor with a porphyrin-based luminescent metal–organic framework (LMOFs) which exhibited a dual-mode response to trace amounts of mercury ions (Hg2+) has been successfully constructed. The porous LMOF probe was fabricated using a simple solvothermal reaction, and assembled with Zr–O clusters as inorganic nodes and meso-tetra(4-carboxyphenyl)porphyrin (TCPP) ligands as organic bridging struts. The sensing activity was realized by using the inherent TCPP struts as recognition sites and signal reporter, which presented a specific interaction with Hg2+ over other potentially interfering metallic cations. The LMOFs which were developed exhibited a visible fluorescent quenching (bright red-dark red) and a colorimetric response (purple-light green) in the presence of Hg2+ with a response rate as rapid as 2 min. Additionally, the fluorescence quenching showed a linear correlation in the Hg2+ concentration range from 0.1 to 10 μM and the limit of detection was calculated to be 6 nM, which was in agreement with the acceptable level of Hg2+ in drinking water mandated by the United States Environmental Protection Agency. Furthermore, the sensor for Hg2+ detection was reversible after the treatment with potassium iodide solution, which suggested that it has great potential as an economical alternative for practical Hg2+ quantification in water. The easily constructed sensory probe was also applied to determine the concentration of Hg2+ in tap water to demonstrate its practical applications.
Introduction
As a highly toxic and bio-accumulative heavy metal, mercury species present severe threats to environmental safety and human health.1,2 Unfortunately, the divalent mercury ions (Hg2+), released from natural sources and industrial by-products, are widespread in ground and subsurface waters.3,4 Because of the growing awareness of Hg2+ toxicity even at a very low level, considerable efforts have been devoted to developing convenient and reliable analytical methods for Hg2+ detection. Although some traditional strategies have been exploited for the accurate and sensitive determination of Hg2+, the requirement of expensive instrumentation and relatively complex processes limits their wide application for in-field detection in water samples.5 Therefore, a simple, low cost and rapid method such as fluorescence and/or colorimetric chemosensor seems more attractive because of their visual readout, simple operation and rapid response rate.6–19Luminescent metal–organic frameworks (LMOFs) have recently attracted increasing attention because of their diverse application potential, especially in the chemical sensing fields.20–28 By fine tuning the textural parameters and chemical environment of the pore channels, a delicately designed LMOF can work to selectively detect small molecules,29–34 and even to distinguish structurally similar analytes.35,36 Up to now, several research groups have studied the utilization of LMOFs for the specific detection of Hg2+ and methylmercury.37–44 As reported in these research projects, the uncoordinated pyridyl nitrogen atoms in the organic linker were usually employed as Hg2+ recognition sites and lanthanide/transition metals such as europium ions (Eu3+), terbium ions (Tb3+) and zinc ions (Zn2+) in the framework were designed as the fluorescent reporter.39–43 However, the lack of excellent hydrolytic stability or channel shrinkage in the condensed MOFs in aqueous solution has confined their sensing process to organic media.39,43 It seems that exploring a chemically stable LMOF is preferred for the in-field detection of Hg2+ in water samples.37,38,42 Meanwhile, optical sensing assays with a colorimetric and fluorimetric dual mode of operation could offer more than one kind of output signal simply and rapidly, thus making the detection results more convincing although use of such a dual mode MOF-based sensor remains rather limited.45,46 This strongly encouraged us to explore a robust and chemically stable LMOF, which could be characterized with a dual mode of fluorimetric and colorimetric response for practical Hg2+ detection.
It has been documented that zirconium (Zr)-based MOFs with porphyrinic struts not only possess exceptional chemical and hydrolytic stability,47–49 but also have potential in optical sensing thanks to the unique spectral and electronic features of the porphyrin moiety. Compared with other heterogeneous carriers, porous porphyrin-based LMOFs integrate with the inherent signal recognition units of porphyrin molecules to act as an organic linker to bridge the inorganic node so that no complicated procedures for the introduction of porphyrin recognition sites are needed. However, there are still very few such chemosensors and there is no report on their application for Hg2+ detection.45,46 Therefore, it is important to explore porphyrin-based LMOFs for specific Hg2+ recognition in aqueous solution.
In this research, meso-tetra(4-carboxyphenyl)porphyrin (TCPP) molecules were used as bridging struts, recognition sites and signal reporters to construct the robust PCN-224 sensor (Scheme 1). The macrocycle of the TCPP units containing four nitrogens could rapidly interact with Hg2+ which influenced the electronic structure of the porphyrin plane, leading to the variation of spectroscopic properties.7 As expected, the porous PCN-224 probe exhibits an obvious fluorescent and colorimetric response which correlated with the applied Hg2+ level because of the formation of a TCPP–Hg2+ complex. The dual readout sensing system combined the high sensitivity of the fluorescent assay and the convenience and low cost of the visual assay. The response rate was as rapid as 2 min, and the quenching constant (KSV) for Hg2+ was calculated to be 6.4 × 105 M−1, which was much higher than those in the reported work by Wang et al.40 Furthermore, the dual mode sensing system for Hg2+ was scarcely affected by other potentially interfering metallic cations and was reversible by the treatment with potassium iodide (KI) solution. The easily constructed sensory probe was also applied to determine the concentration of Hg2+ in tap water to illustrate its practical application.
Experimental details
Chemicals and materials
TCPP and mercury(II) nitrate monohydrate [Hg(NO3)2·H2O] standard solution (1 mg mL−1) were obtained from J&K Scientific Ltd, China. 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) was supplied by Sigma-Aldrich. Zirconyl chloride octahydrate (ZrOCl2·8H2O), acetic acid, N,N-dimethylformamide (DMF), KI, sodium chloride, potassium chloride, calcium nitrate tetrahydrate [Ca(NO3)2·4H2O], nickel(II) nitrate hexahydrate [Ni(NO3)2·6H2O], copper(II) nitrate trihydrate [Cu(NO3)2·3H2O], zinc nitrate hexahydrate [Zn(NO3)2·6H2O], aluminium nitrate monohydrate [Al(NO3)3·H2O], magnesium nitrate hexahydrate [Mg(NO3)2·6H2O] and iron(III) nitrate nonahydrate [Fe(NO3)3·9H2O] were purchased from Sinopharm Chemical Reagent Co., Ltd, China. All reagents were of analytical grade and used as received without additional purification. All water (18.1 MΩ cm−1) was obtained using a NW Ultra-pure Water System (Heal Force, China) and used for all the experiments.Synthesis of PCN-224
PCN-224 microcrystals were synthesized in a moderate solvothermal condition as reported with a slight modification.50 Briefly, ZrOCl2·8H2O (312.5 mg, 0.97 mmol), TCPP (62.5 mg, 0.08 mmol) and acetic acid (62.5 mL) were dissolved in DMF (125 mL) with ultrasonic mixing for 30 min. The mixed solution was placed in a 250 mL vial and heated at 65 °C for three days. After cooling to room temperature, the purple solid, which was PCN-224, was collected by centrifugation and washed three times with DMF. The obtained solid was soaked in DMF for 12 h to remove the unreacted TCPP ligand, and then soaked with acetone repeatedly at 65 °C to replace the trapped DMF. Finally, the as-synthesized PCN-224 particles were obtained by activation at 120 °C in vacuo for 24 h.Fluorescent and colorimetric sensing of Hg2+
The stock solution of PCN-224 (50 mg L−1) was prepared by dispersing PCN-224 particles in HEPES buffer solution (60 mM, pH = 7.0). Then, Hg2+ standard solutions were added to the PCN-224 suspension. The final Hg2+ concentrations were in the range from 0.1 to 10 μM, and the final volumes of the prepared batches of suspension were kept constant at 2 mL. After incubation at room temperature for approximately 2 min, the fluorescence spectra of the sensing systems were recorded with an emission wavelength in the range from 590 to 750 nm (λex = 430 nm). The ultraviolet-visible (UV-Vis) absorption spectra of the PCN-224 suspension containing different concentrations of Hg2+ were also measured at a wavelength range from 350 to 700 nm.To investigate the response kinetics, three different samples with Hg2+ concentrations of 0, 1 or 5 μM were prepared by adding Hg2+ standard solution to PCN-224 suspension in 60 mM HEPES buffer solution (pH = 7.0). The fluorescence intensities of the resultant mixtures were recorded at different time intervals from 30 to 600 s with excitation at 430 nm.
To study the interfering effects of the potentially co-existent metallic cations, a series of metallic ion solutions were added into the fluorescent probe suspension. The concentrations of the metallic ions were set at 5 μM in the presence or absence of Hg2+ (5 μM). The fluorescence and absorption measurements were conducted under the same conditions as the preceding sensing experiments.
Reversible determination for Hg2+
The reversibility of the PCN-224 sensor for Hg2+ sensing was measured using repeated additions of KI solution. Specifically, 10 μM KI (2 equiv. to a Hg2+ concentration of 5 μM) was added to the PCN-224 sensing system for 10 min to regenerate the fluorescent emission, and then the fluorescence spectra was measured. The reversibility of color change was directly detected by the naked eye and recorded with a digital camera. The reversible processes of Hg2+ sensing/KI regeneration were repeated for four cycles.The resulting particles of PCN-224 were collected by centrifugation and washed three times with water and DMF. The regenerated PCN-224 microcrystals were denoted as PCN-224-KI, and then characterized using powder X-ray diffraction (XRD) and scanning electron microscopy (SEM) to follow the change of structural integrity.
Recovery experiments of Hg2+ in various water samples
The recovery experiments were employed to evaluate the Hg2+ content in real tap water, distilled water and river water samples. Under the standard addition process, different concentrations of Hg2+ (0, 0.5 and 2 μM) were added into real water samples and then determined with a fluorescence measurement. The pH of the water samples was adjusted to pH = 7 by the addition of HEPES buffer. The detected Hg2+ concentrations were calculated by the fluorescent response (λem = 652 nm) of spiked water samples and linear regression equations. All the experiments were repeated four times to get an average value of the detected Hg2+ concentration. Then, the recovery percentages were calculated to evaluate the degree of deviation of the detected value compared to the amount of added Hg2+.Instruments and methods
Fluorescence and UV-Vis absorption spectra were measured with a RF-5301PC spectrofluorophotometer (Shimadzu, Japan) and a UV-2550 spectrophotometer (Shimadzu, Japan), respectively. The powder XRD patterns were acquired with a D8 Advance diffractometer (Bruker) using Cu Kα radiation (40 kV, 40 mA) at a scan rate of 6° min−1 over the range of 2–20° (2θ). Nitrogen (N2) sorption isotherms were recorded using a TriStar 3020 surface area and pore size analyzer (Micromeritics). All the samples were degassed under vacuum for 12 h before the measurements were made. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method using adsorption data at a relative pressure (P/P0) lower than 0.15. Using density functional theory (DFT), the pore size distributions were derived from the adsorption data. Field emission scanning electron microscopy was performed with a JSM-6700F electron microscope (JEOL). Thermogravimetric analysis (TGA) was conducted on a thermogravimetric analyzer (PerkinElmer) by heating the sample to 600 °C under an air atmosphere (50 mL min−1) at a heating rate of 10 °C min−1.Results and discussion
Characterization of PCN-224 particles
The highly crystallized PCN-224, assembled with Zr–O clusters and TCPP ligands, was prepared using a moderate solvothermal reaction. The as-synthesized PCN-224 exhibits a typical Im3m space group which is in agreement with the reported topology as verified by powder XRD (Fig. 1a).48 The SEM image illustrates that the discrete particles of PCN-224 have a cubic shape, and the regular particle size is in the micrometer scale of around 2 μm (Fig. S1A, ESI†). Additionally, the particle size of PCN-224 was also measured using the dynamic light scattering (DLS) technique. The hydrodynamic size is narrowly distributed and the peak appeared at approximately 1865 nm (Fig. S2, ESI†), indicating their good water dispersity and homogeneity. As shown in Fig. S3A,† a typical type-I isotherm for the as-synthesized PCN-224 was revealed by the N2 sorption measurement. A high BET surface area, calculated to be 2489 m2 g−1, matched well with the reported value (2600 m2 g−1).48 Pore size was determined to be 1.85 nm using the DFT method (Fig. S3B, ESI†). The TGA data also show that PCN-224 is stable in air up to 350 °C (Fig. S4, ESI†), and has a weight loss of about 53% upon heating from 350 to 600 °C which corresponded with the decomposition of the TCPP linkers. Because porphyrin containing TCPP is an inherent component as the organic struts for the construction of the open framework of PCN-224, such a high porphyrin content in the PCN-224 is reasonable. With abundant fluorogenic and chromogenic porphyrin recognition sites in three-dimensional large open channels, PCN-224 meets the critical prerequisite as an ideal platform for aqueous phase Hg2+ sensing.Kinetics for the fluorescent sensing
To investigate the response rate of the current fluorescent probe to the analyte, the kinetic characteristics for Hg2+ sensing was measured at different time intervals (Fig. 2). Upon the addition of Hg2+ (1 μM), the fluorescent intensity of PCN-224 suspension starts to decrease immediately and reached an almost minimum value and stability after 120 seconds, which indicated that the reaction is already complete. It was also found that a higher concentration (5 μM) of Hg2+ lead to a shorter equilibrium time of approximate 60 seconds. Therefore, the response time of the PCN-224 probe for Hg2+ was determined to be as short as 120 seconds, which was set as the incubation time for the Hg2+ detection in all the following experiments.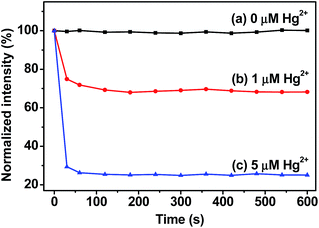 | ||
| Fig. 2 Effect of response time on the fluorescent intensities upon the addition of Hg2+ into the PCN-224 suspension. The concentrations of Hg2+ were set as (a) 0, (b) 1 and (c) 5 μM. | ||
Fluorescent detection of Hg2+
To gain an insight into the sensing properties of PCN-224 towards Hg2+, fluorescent quenching titration with different amounts of Hg2+ were conducted with an excitation wavelength of 430 nm. As shown in Fig. 3A, the fluorescence spectrum of PCN-224 suspension in the absence of Hg2+ showed a strong emission peak at 651 nm and a weak shoulder at 705 nm, which is characteristic of the fluorescent emission from free porphyrin units. Upon the addition of Hg2+, the fluorescent emission from PCN-224 was obviously quenched in a Hg2+ concentration dependent way. An initial fluorescence change could be recognized at Hg2+ concentrations as low as 0.1 μM, which confirmed the significant sensitivity of the investigated probe. The initial fluorescent intensity of PCN-224 was effectively quenched to about 14% at the Hg2+ concentration of 10 μM, which confirmed the capability of Hg2+ for the efficient quenching of the fluorescent emission from the PCN-224 suspension. The reason for the fluorescent quenching can be ascribed to the fact that Hg2+ is specifically bound to the nitrogen atoms of TCPP units, leading to the reverse photoinduced electron transfer.51,52 The inset in Fig. 3B shows the photographs of PCN-224 suspension in the presence of different amounts of Hg2+ illuminated by a UV-lamp (λex = 365 nm). The bright red emission of PCN-224 suspension becomes darker and darker upon the addition of an increasing quantity of Hg2+, which highlights the feasibility of naked eye detection of Hg2+ in aqueous solution.In general, the fluorescent quenching could be quantitatively described by the Stern–Volmer equation:
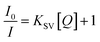 | (1) |
Colorimetric detection of Hg2+
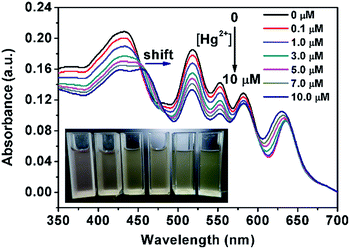
To exploit the possibility of detecting Hg2+ with visual colour change, the absorption spectra of PCN-224 suspension were recorded (Fig. 4). In the absence of Hg2+, the UV-Vis absorption spectra of PCN-224 suspension presented a strong visible Soret band at 430 nm and four Q bands at 518, 552, 584 and 637 nm, which were ascribed to the characteristic absorption peaks of porphyrin units.35 Upon the addition of Hg2+ from 0 to 10 μM, the Soret band of PCN-224 at 430 nm gradually decreased in intensity, even when a small amount of Hg2+ (0.1 μM, red curve) was added, implying the onset of coordinative interactions between the porphyrin core and Hg2+.17 The Soret band is also associated with a bathochromic shift at higher concentration of Hg2+. When more than 10 μM of Hg2+ was added, the Soret band of PCN-224 breaks into two separate peaks at 430 and 455 nm. The occurrence of absorption at 455 nm confirmed the formation of a TCPP–Hg complex,54 because metalloporphyrin usually absorbs at a longer wavelength with a smaller extinction coefficient when compared to that of a metal-free porphyrin.15,17 The complexation process also leads to notable colour variation upon the addition of an increasing amount of Hg2+. As shown in the inset of Fig. 4, the colour of the PCN-224 suspension gradually changed from purple to light green with the formation of the charge-transfer complex between Hg2+ and porphyrin units.17 This concentration dependent colorimetric response could be easily distinguished by the naked eye, which may allow the simple and rapid on-site detection of Hg2+ in water samples.Selectivity of the fluorimetric and colorimetric assay
To evaluate the potential of PCN-224 as an ion-selective sensor for Hg2+ detection, the fluorescent and colorimetric responses in the presence of various environmentally relevant metallic cations, including aluminium (Al3+), calcium (Ca2+), copper (Cu2+), iron (Fe3+), magnesium (Mg2+), nickel (Ni2+), potassium (K+), sodium (Na+), and zinc (Zn2+), were recorded in the assay. As shown in Fig. 5 (blue bar), insignificant changes in the fluorescent emission are observed when a serious of metallic ions were added to the PCN-224 suspension. In contrast, the emission of the PCN-224 suspension was greatly quenched in the presence of Hg2+, which could be directly observed by the naked eye under UV lamp illumination (Fig. 5B). Because the high specificity is crucial for most probes to be applied in the real sample detection, other possibly co-existent metallic cations were further added to the PCN-224-Hg2+ detection system. The response of the PCN-224 suspension towards Hg2+ was almost unaffected in a background of environmentally relevant metallic ions (Fig. 5A, red bar). These competition experiments showed that the PCN-224 sensor is rather difficult to complex with nonspecific ions, justifying its potential for selective Hg2+ recognition.To minimize the possible false-positive responses and improve selectivity, a two signal sensing strategy can be of great assistance in the accurate recognition of the target analytes. In this work, the colorimetric change could provide an additional sensing signal to avoid the possible interference in the fluorescence quenching. The inspection of the UV-Vis spectra (Fig. 6) of the PCN-224 suspension upon the addition of various cations other than Hg2+ does not reveal any evident perturbation of the porphyrin electronic states. The Soret band remains virtually unchanged and the colours of PCN-224 suspensions remain purple in the presence of potentially interfering metallic cations (Fig. 6B). In contrast, the colour of PCN-224 suspension containing 5 μM Hg2+ clearly becomes light green which is visible to the naked eye, and an obvious bathochromic shift to 455 nm in the Soret band could be observed. Therefore, the fluorogenic and chromogenic PCN-224 probe is effective for selective Hg2+ detection with an excellent anti-interfering ability against the potentially co-existent metallic cations.
To gain an insight into the interaction mechanism between Hg2+ ions and porphyrin, the UV-Vis spectra of free TCPP molecules in DMF/HEPES buffer (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7) were also measured upon the addition of Hg2+. As shown in Fig. S6A (ESI†), the Soret band of TCPP gradually disappeared at 415 nm, and a red-shifted Soret band was generated at 443 nm, indicating the formation of a TCPP–Hg complex.54 The clear isosbestic point at 426 nm showed the gradual transformation of two species between the TCPP molecule and the TCPP–Hg complex in the sensing process.54 Simultaneously, the rapid formation of the TCPP–Hg complex between Hg2+ and the porphyrin plane increased the symmetry of the macrocycle so that four Q-bands of TCPP were reduced to two bands at 582 nm and 626 nm (Fig. S6B, ESI†).15 It has been reported that the TCPP–Hg complex should be a “sitting-atop” (SAT) structure.55–57 Because the dimension of the mercury atom is larger than that of the porphyrin ring, Hg2+ should be located out of the plane of the porphyrin ring, distorting the porphyrin nucleus (Fig. S6A, ESI†). This reaction is relatively easier and faster for forming such a SAT complex compared with other representative metal ions.58 In contrast, the incorporation reaction of transition metal ions into the porphyrin ring is generally very slow and may need additional heating because of the difficulty in inserting them into the porphyrin ring.58,59 Taken together, the previously mentioned mechanisms might result in the formation of a nonfluorescent TCPP–Hg complex and rationalize the selective detection of Hg2+ using the current PCN-224 probe.
1, pH = 7) were also measured upon the addition of Hg2+. As shown in Fig. S6A (ESI†), the Soret band of TCPP gradually disappeared at 415 nm, and a red-shifted Soret band was generated at 443 nm, indicating the formation of a TCPP–Hg complex.54 The clear isosbestic point at 426 nm showed the gradual transformation of two species between the TCPP molecule and the TCPP–Hg complex in the sensing process.54 Simultaneously, the rapid formation of the TCPP–Hg complex between Hg2+ and the porphyrin plane increased the symmetry of the macrocycle so that four Q-bands of TCPP were reduced to two bands at 582 nm and 626 nm (Fig. S6B, ESI†).15 It has been reported that the TCPP–Hg complex should be a “sitting-atop” (SAT) structure.55–57 Because the dimension of the mercury atom is larger than that of the porphyrin ring, Hg2+ should be located out of the plane of the porphyrin ring, distorting the porphyrin nucleus (Fig. S6A, ESI†). This reaction is relatively easier and faster for forming such a SAT complex compared with other representative metal ions.58 In contrast, the incorporation reaction of transition metal ions into the porphyrin ring is generally very slow and may need additional heating because of the difficulty in inserting them into the porphyrin ring.58,59 Taken together, the previously mentioned mechanisms might result in the formation of a nonfluorescent TCPP–Hg complex and rationalize the selective detection of Hg2+ using the current PCN-224 probe.
Reversible sensing for Hg2+
To evaluate the reversibility of the PCN-224 probe for Hg2+ sensing, KI solution was utilized as a disassociation agent for the removal of Hg2+ from the sensing system. As shown in Fig. 7, when KI (2 equiv. to Hg2+) is added to the PCN-224-Hg2+ suspension, the fluorescent emission at 652 nm reverts to 89% of the initial intensity and the colour also recovers from light green back to purple. This might be attributed to a much stronger binding ability of I− to Hg2+, and indicates that the TCPP recognition units in PCN-224 is regenerated because of the dissociation of Hg2+ from the TCPP–Hg2+ complex by I−.60–62 In addition, when Hg2+ (5 μM) was added to the previous suspension again, the emission at 652 nm was immediately quenched and the colour of PCN-224 suspension turned light green. This reversible process was repeated for four cycles, showing the reversibility of the PCN-224 sensor for Hg2+ detection. Furthermore, the structure properties of PCN-224-KI were tracked using XRD and SEM. Even after regeneration by the KI solution, the PCN-224 microcrystal retains its high crystallinity as verified by the powder XRD pattern (Fig. S7, ESI†), and the morphology integrity is maintained as illustrated by the SEM image (Fig. S1B, ESI†), which should benefit from the excellent chemical and hydrolytic stability of the currently used LMOFs. The good reusability of PCN-224 MOFs suggests that it shows great promise as a heterogeneous sensor for Hg2+ detection.Recovery in various water samples
To evaluate the impact of a real water environment, the practicality of the explored probe for the detection of Hg2+ was also tested in tap, distilled and river water samples. As shown in Table 1, no Hg2+ was detected in the blank water samples. Then, known amounts of Hg2+ at different concentrations were introduced into various water samples to perform a recovery test. The resultant recoveries of measurements varied from 96% to 104%, and exhibited a good agreement between experimental and real values. These results suggest that the developed PCN-224 sensor is applicable to the detection of Hg2+ in natural water samples.| Samples | Spiked values (μM) | Detected values (μM) | RSDc (μM) | Recoveryd (%) |
|---|---|---|---|---|
| a ND is not detected.b n is the repetitive measurement number.c Relative standard deviations (RSD) are calculated based on measurements repeated 4 times.d Recovery (%) = 100 × (Cmean detected/Cspiked Hg2+). | ||||
| Tap water | 0 | ND | ND | ND |
| 0.50 | 0.51 | 0.02 | 102.0 | |
| 2.00 | 1.94 | 0.06 | 97.0 | |
| Distilled water | 0 | ND | ND | ND |
| 0.50 | 0.48 | 0.02 | 96.0 | |
| 2.00 | 1.98 | 0.04 | 99.0 | |
| River water | 0 | ND | ND | ND |
| 0.50 | 0.52 | 0.03 | 104.0 | |
| 2.00 | 2.07 | 0.05 | 103.5 | |
In the past few years, many strategies have been successfully developed for the detection of Hg2+ based on fluorescent and colorimetric methods. As shown in Table S1 (ESI†), the sensing features of the PCN-224 probe are comparable to the majority of previously reported MOF-based and porphyrin-based sensors. Additionally, the proposed method is very simple without multi-step modification procedures to introduce the porphyrin recognition sites into the heterogeneous carrier. However, some of the reported sensors also suffer from irreversibility,10,17 whereas the proposed sensor can be easily be regenerated by dissociation of Hg2+ with KI solution.
Conclusions
In summary, porphyrinic PCN-224 has been successfully developed for fluorescent and colorimetric determination of Hg2+ in aqueous solutions. The TCPP struts in PCN-224 were strategically designed as recognition sites and as a signal reporter. The newly devised probe exhibited an obvious fluorescent and colorimetric response correlated with the applied Hg2+ level, and combined the high sensitivity of the fluorescent assay and with the convenience and low cost of the visual assay. The response rate was as rapid as 2 min, and the LOD was calculated to be 6 nM, which is below the acceptable level of Hg2+ in drinking water mandated by the EPA. Furthermore, the dual readout Hg2+ sensing system was scarcely affected by other potentially interfering metallic cations and was reversible by treatment with KI solution. The easily constructed sensory probe was successfully applied to determine the concentration of Hg2+ in tap water. Given the ready installation of the exposed metal sites in the porphyrin center,63 this strategy may be easily expanded for the design of rapid and specific porphyrin-based sensors for other analytes with different metallic ions in fluorescent porphyrin molecules, and this research is still in progress.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (51072053, 51372084), the Innovation Program of Shanghai Municipal Education Commission (13zz040), and the 111 Project (B14018).References
- Y. Si, X. Wang, Y. Li, K. Chen, J. Wang, J. Yu, H. Wang and B. Ding, J. Mater. Chem. A, 2014, 2, 645–652 CAS.
- N. Vasimalai and S. A. John, J. Mater. Chem. A, 2013, 1, 4475–4482 CAS.
- S. Jayabal, R. Sathiyamurthi and R. Ramaraj, J. Mater. Chem. A, 2014, 2, 8918–8925 CAS.
- A. Senthamizhan, A. Celebioglu and T. Uyar, J. Mater. Chem. A, 2014, 2, 12717–12723 CAS.
- C. Pécheyran, C. R. Quetel, F. M. M. Lecuyer and O. F. X. Donard, Anal. Chem., 1998, 70, 2639–2645 CrossRef PubMed.
- G. R. You, S. Y. Lee, J. J. Lee, Y. S. Kim and C. Kim, RSC Adv., 2016, 6, 4212–4220 RSC.
- Y. W. Choi, J. J. Lee, G. R. You and C. Kim, RSC Adv., 2015, 5, 38308–38315 RSC.
- H. Xu, S. Huang, C. Liao, Y. Li, B. Zheng, J. Du and D. Xiao, RSC Adv., 2015, 5, 89121–89127 RSC.
- M. Shamsipura, M. Sadeghi, M. Beyzavi and H. Sharghi, Mater. Sci. Eng., C, 2015, 48, 424–433 CrossRef PubMed.
- Y. Wang and F. Wu, Polymer, 2015, 56, 223–228 CrossRef CAS.
- G.-H. Chen, W.-Y. Chen, Y.-C. Yen, C.-W. Wang, H.-T. Chang and C.-F. Chen, Anal. Chem., 2014, 86, 6843–6849 CrossRef CAS PubMed.
- C.-Y. Li, X.-B. Zhang, L. Qiao, Y. Zhao, C.-M. He, S.-Y. Huan, L.-M. Lu, L.-X. Jian, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2009, 81, 9993–10001 CrossRef CAS PubMed.
- Y. Yang, J. Jiang, G. Shen and R. Yu, Anal. Chim. Acta, 2009, 636, 83–88 CrossRef CAS PubMed.
- Z. Fang and B. Liu, Tetrahedron Lett., 2008, 49, 2311–2315 CrossRef CAS.
- Z. Fang, K.-Y. Pu and B. Liu, Macromolecules, 2008, 41, 8380–8387 CrossRef CAS.
- X.-J. Zhu, S.-T. Fu, W.-K. Wong, J.-P. Guo and W.-Y. Wong, Angew. Chem., 2006, 118, 3222–3226 CrossRef.
- T. Balaji, M. Sasidharan and H. Matsunaga, Analyst, 2005, 130, 1162–1167 RSC.
- X.-B. Zhang, C.-C. Guo, Z.-Z. Li, G.-L. Shen and R.-Q. Yu, Anal. Chem., 2002, 74, 821–825 CrossRef CAS PubMed.
- W. Chana, R. Yang and K. Wang, Anal. Chim. Acta, 2001, 444, 261–269 CrossRef.
- J. Zhou, H. Li, H. Zhang, H. Li, W. Shi and P. Cheng, Adv. Mater., 2015, 27, 7072–7077 CrossRef CAS PubMed.
- Y. Cui, B. Chen and G. Qian, Coord. Chem. Rev., 2014, 273–274, 76–86 CrossRef CAS.
- Z. Dou, J. Yu, Y. Cui, Y. Yang, Z. Wang, D. Yang and G. Qian, J. Am. Chem. Soc., 2014, 136, 5527–5530 CrossRef CAS PubMed.
- D. Liu, K. Lu, C. Poon and W. Lin, Inorg. Chem., 2014, 53, 1916–1924 CrossRef CAS PubMed.
- R.-B. Lin, F. Li, S.-Y. Liu, X.-L. Qi, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2013, 52, 13429–13433 CrossRef CAS PubMed.
- Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. V. Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., Int. Ed., 2009, 48, 500–503 CrossRef CAS PubMed.
- B. Chen, Y. Yang, F. Zapata, G. Lin, G. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693–1696 CrossRef CAS.
- A. V. Desai, P. Samanta, B. Manna and S. K. Ghosh, Chem. Commun., 2015, 51, 6111–6114 RSC.
- S. S. Nagarkar, A. V. Desai and S. K. Ghosh, Chem.–Eur. J., 2015, 21, 9994–9997 CrossRef CAS PubMed.
- B. J. Deibert and J. Li, Chem. Commun., 2014, 50, 9636–9639 RSC.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- Z. Hu, K. Tan, W. P. Lustig, H. Wang, Y. Zhao, C. Zheng, D. Banerjee, T. J. Emge, Y. J. Chabal and J. Li, Chem. Sci., 2014, 5, 4873–4877 RSC.
- S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., 2013, 125, 2953–2957 CrossRef.
- J. Yang, Z. Wang, K. Hu, Y. Li, J. Feng, J. Shi and J. Gu, ACS Appl. Mater. Interfaces, 2015, 7, 11956–11964 CAS.
- Z. Hu, S. Pramanik, K. Tan, C. Zheng, W. Liu, X. Zhang, Y. J. Chabal and J. Li, Cryst. Growth Des., 2013, 13, 4204–4207 CAS.
- P. Wu, Y. Liu, Y. Liu, J. Wang, Y. Li, W. Liu and J. Wang, Inorg. Chem., 2015, 54, 11046–11048 CrossRef CAS PubMed.
- L. Wen, X. Zheng, K. Lv, C. Wang and X. Xu, Inorg. Chem., 2015, 54, 7133–7135 CrossRef CAS PubMed.
- Y.-M. Zhu, C.-H. Zeng, T.-S. Chu, H.-M. Wang, Y.-Y. Yang, Y.-X. Tong, C.-Y. Su and W.-T. Wong, J. Mater. Chem. A, 2013, 1, 11312–11319 CAS.
- H.-M. Wang, Y.-Y. Yang, C.-H. Zeng, T.-S. Chu, Y.-M. Zhu and S. W. Ng, Photochem. Photobiol. Sci., 2013, 12, 1700–1706 CAS.
- F. Xu, L. Kou, J. Jia, X. Hou, Z. Long and S. Wang, Anal. Chim. Acta, 2013, 804, 240–245 CrossRef CAS PubMed.
- A. Shahat, H. M. A. Hassana and H. M. E. Azzazy, Anal. Chim. Acta, 2013, 793, 90–98 CrossRef CAS PubMed.
- J. Jia, F. Xu, Z. Long, X. Hou and M. J. Sepaniak, Chem. Commun., 2013, 49, 4670–4672 RSC.
- J. He, K.-K. Yee, Z. Xu, M. Zeller, A. D. Hunter, S. S.-Y. Chui and C.-M. Che, Chem. Mater., 2011, 23, 2940–2947 CrossRef CAS.
- M. Yuan, Y. Li, J. Li, C. Li, X. Liu, J. Lv, J. Xu, H. Liu, S. Wang and D. Zhu, Org. Lett., 2007, 9, 2313–2316 CrossRef CAS PubMed.
- H. Wang, Y. Wang, J. Jin and R. Yang, Anal. Chem., 2008, 80, 9021–9028 CrossRef CAS PubMed.
- D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., 2012, 124, 10453–10456 CrossRef.
- D. Feng, W.-C. Chung, Z. Wei, Z.-Y. Gu, H.-L. Jiang, Y.-P. Chen, D. J. Darensbourg and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 17105–17110 CrossRef CAS PubMed.
- H.-L. Jiang, D. Feng, K. Wang, Z.-Y. Gu, Z. Wei, Y.-P. Chen and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934–13938 CrossRef CAS PubMed.
- W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef CAS PubMed.
- Y. Cho, S. S. Lee and J. H. Jung, Analyst, 2010, 135, 1551–1555 RSC.
- L. Sun, Y. Li, M. Sun, H. Wang, S. Xu, C. Zhang and Q. Yang, New J. Chem., 2011, 35, 2697–2704 RSC.
- J. Hatai, S. Pal, G. P. Jose and S. Bandyopadhyay, Inorg. Chem., 2012, 51, 10129–10135 CrossRef CAS PubMed.
- O. Horvath, Z. Valicsek and A. Vogler, Inorg. Chem. Commun., 2004, 7, 854–857 CrossRef CAS.
- S. Bettini, R. Pagano, L. Valli and G. Giancane, J. Phys. Chem. C, 2014, 118, 12384–12390 CAS.
- N. Motreff, S. L. Gac, M. Luhmer, E. Furet, J.-F. Halet, T. Roisnel and B. Boitrel, Angew. Chem., Int. Ed., 2011, 50, 1560–1564 CrossRef CAS PubMed.
- M.-C. Wang, L.-S. Sue, B.-C. Liau, B.-T. Ko, S. Elango and J.-H. Chen, Inorg. Chem., 2001, 40, 6064–6068 CrossRef CAS PubMed.
- M. Biesaga, K. Pyrzynska and M. Trojanowicz, Talanta, 2000, 51, 209–224 CrossRef CAS PubMed.
- K. Kilian and K. Pyrzynska, Talanta, 2003, 60, 669–678 CrossRef CAS PubMed.
- S. Goswami, A. K. Das and S. Maity, Dalton Trans., 2013, 42, 16259–16263 RSC.
- J. Weng, Q. Mei, Q. Ling, Q. Fan and W. Huang, Tetrahedron, 2012, 68, 3129–3134 CrossRef CAS.
- A. K. Mahapatra, J. Roy, P. Sahoo, S. K. Mukhopadhyay and A. Chattopadhyay, Org. Biomol. Chem., 2012, 10, 2231–2236 CAS.
- J. Yang, Z. Wang, Y. Li, Q. Zhuang and J. Gu, Chem. Mater., 2016, 28, 2652–2658 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of PCN-224 and PCN-224-Hg2+/KI, N2 sorption isotherms, thermogravimetric-differential thermal analysis (TG-DTA) profile, calculation for the limit of detection, powder XRD patterns of PCN-224-KI. See DOI: 10.1039/c6ra13766k |
| This journal is © The Royal Society of Chemistry 2016 |