Solventless synthesis of Ru(0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis†
Abstract
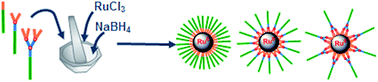
Ruthenium nanoparticles (NPs) are prepared by milling under air ruthenium chloride (RuCl3), sodium borohydride (NaBH4) and a polyphosphorhydrazone (PPH) dendron (generation 0 to 2) having an alkyl chain at the focal point and triarylphosphines on the surface. The resulting NPs have a diameter in the 2 to 3 nm range and they are stable upon storage in solution or as powders. They can efficiently catalyze hydrogenation of styrene. The interaction between the dendrons and the NPs is studied, and the influence of the alkyl chain length and dendron generation is also discussed.

 Please wait while we load your content...
Please wait while we load your content...