Novel synthesised flavone derivatives provide significant insight into the structural features required for enhanced anti-proliferative activity†
Abstract
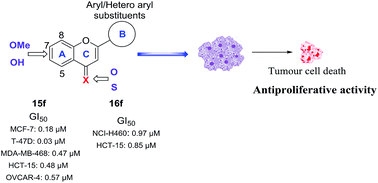
With many cancers showing resistance to current chemotherapies, the search for novel anti-cancer agents is attracting considerable attention. Natural flavonoids have been identified as useful leads in such programmes. However, since an in-depth understanding of the structural requirements for optimum activity is generally lacking, further research is required before the full potential of flavonoids as anti-proliferative agents can be realised. Herein a broad library of 76 methoxy and hydroxy flavones, and their 4-thio analogues, was constructed and their structure–activity relationships for anti-proliferative activity against the breast cancer cell lines MCF-7 (ER +ve), MCF-7/DX (ER +ve, anthracycline resistant) and MDA-MB-231 (ER −ve) were probed. Within this library, 42 compounds were novel, and all compounds were afforded in good yields and >95% purity. The most promising lead compounds, specifically the novel hydroxy 4-thioflavones 15f and 16f, were further evaluated for their anti-proliferative activities against a broader range of cancer cell lines by the National Cancer Institute (NCI), USA and displayed significant growth inhibition profiles (e.g. compound-15f: MCF-7 (GI50 = 0.18 μM), T-47D (GI50 = 0.03 μM) and MDA-MB-468 (GI50 = 0.47 μM) and compound-16f: MCF-7 (GI50 = 1.46 μM), T-47D (GI50 = 1.27 μM) and MDA-MB-231 (GI50 = 1.81 μM)). Overall, 15f and 16f exhibited 7–46 fold greater anti-proliferative potency than the natural flavone chrysin (2d). A systematic structure–activity relationship study against the breast cancer cell lines highlighted that free hydroxyl groups and the B-ring phenyl groups were essential for enhanced anti-proliferative activities. Substitution of the 4-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality with a 4-C
O functionality with a 4-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functionality, and incorporation of electron withdrawing groups at C-4′ of the B-ring phenyl, also enhanced activity. Molecular docking and mechanistic studies suggest that the anti-proliferative effects of flavones 15f and 16f are mediated via ER-independent cleavage of PARP and downregulation of GSK-3β for MCF-7 and MCF-7/DX cell lines. For the MDA-MB-231 cell line, restoration of the wild-type p53 DNA binding activity of mutant p53 tumour suppressor gene was indicated.
S functionality, and incorporation of electron withdrawing groups at C-4′ of the B-ring phenyl, also enhanced activity. Molecular docking and mechanistic studies suggest that the anti-proliferative effects of flavones 15f and 16f are mediated via ER-independent cleavage of PARP and downregulation of GSK-3β for MCF-7 and MCF-7/DX cell lines. For the MDA-MB-231 cell line, restoration of the wild-type p53 DNA binding activity of mutant p53 tumour suppressor gene was indicated.

 Please wait while we load your content...
Please wait while we load your content...