One-step reduction and simultaneous decoration on various porous substrates: toward oil filtration from water†
Abstract
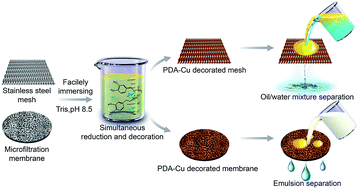
Effective purification of waste water tainted with either immiscible oil/water mixtures or surfactant-stabilized emulsions is always attractive for long-term water remediation. We report here on a versatile development of polydopamine coated copper surfaces on various porous substrates via a simple one-step immersing method. The incorporation of dopamine into this design not only optimizes the operation of preparing simple substances on copper surfaces on a wide range of substrates, but also endows the as-obtained membranes with superhydrophilicity and underwater superoleophobicity. Such membranes are capable of efficiently separating both immiscible oil/water mixtures and surfactant-stabilized emulsions based on the desired substrates, and they display superior chemical resistance to aggressive reagents, excellent recyclability and robust structural stability, making them promising candidates for a variety of oil filtration applications ranging from the treatment of oil/water mixtures to emulsified oily waste water.

 Please wait while we load your content...
Please wait while we load your content...