DOI:
10.1039/C6RA13634F
(Paper)
RSC Adv., 2016,
6, 71287-71294
Hydrophilic modification of PVDF porous membrane via a simple dip-coating method in plant tannin solution†
Received
26th May 2016
, Accepted 9th July 2016
First published on 11th July 2016
Abstract
To improve the hydrophilicity of polyvinylidene fluoride (PVDF) porous membranes, herein, we report for the first time a low-cost and environmental plant tannin coating, which is strongly constructed on the surface of the PVDF membrane via a simple dip-coating method. An oxidation induced aggregation mechanism was detected in the tannin solution through the use of ultraviolet spectrophotometry (UV-Vis). The morphology and chemical composition of the tannin coated PVDF membrane were characterized via field-emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS) and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). After coating with the tannin coating, hydrophilicity and filtration performance are both greatly enhanced, and the modified membranes also have a good emulsion separation performance and excellent antifouling property (after emulsion separation, the flux recovery ratio even reaches 100%). Moreover, these membranes possess outstanding durability that is disclosed by a long-term rinsing experiment. These results indicate that the plant tannin coating has great potential application for the hydrophilic modification of hydrophobic membranes.
1. Introduction
Due to the increased water crisis and environmental awareness worldwide in recent years, water treatment plays a more and more important role and therefore many different methods for water treatment have been developed.1–4 Polymer membrane separation, as an approach for water treatment, has aroused broad attention due to its simple operation, high separation efficiency, absence of phase changes and relatively low energy consumption.5–7 Polyvinylidene fluoride (PVDF), which has excellent properties, such as high mechanical strength, thermal stability, impact resistance, chemical resistance and good film-forming ability comparing with other polymer materials, has been widely used as one of the most promising materials for the fabrication of polymer membranes. However, its low surface energy and lack of hydrogen bonding interaction with water make the PVDF membrane strongly hydrophobic and easily fouled by natural organic matters, which result in a sharp decline in serviceability.8–11 Thus, it is of great significance to improve the hydrophilicity of PVDF membranes for water treatment.
Various methods have been utilized to enhance the hydrophilicity of the PVDF membrane such as physical blending,12–14 polymeric and surface chemical grafting,15–18 and surface chemical treatment.9,19 Compared with most of these methods, surface coating/deposition has attracted increasing interest in recent decades owing to its simplicity, universality and high-efficiency.20,21 However, in regards to porous hydrophobic PVDF membranes, most surface coating materials are weakly adhesive and easily rinsed out during operation and cleaning. Therefore, special coatings, which can firmly and sustainably adhere to the PVDF membrane, should be prepared to transform hydrophobic PVDF membranes into stable and durable highly hydrophilic membranes.
Phenols and polyphenols, which are widely distributed in flora and fauna tissues, have attracted much attention for use in membrane surface engineering due to their strong solid–liquid interfacial activity.22,23 Dopamine, which is rich in catechol groups, has been reported to have stable adhesive ability on many types of substrates via covalent and non-covalent interactions,24 and the preparation procedure is a simple dip-coating method with in situ oxidative polymerization at alkaline pH conditions.25 Many studies have indicated that polydopamine, as a versatile coating, can significantly improve the surface wettability and permeability of some hydrophobic polymer membranes.26–28 Some studies also have shown that the key functional group in dopamine is catechol because it has dramatic chemical versatility and diversity of affinity.25,26,29 However, despite the excellent properties of dopamine, its application is limited by the high cost and the characteristic dark color of polydopamine.30,31 For wider applications, other general materials, which contain catechol and analogous structural units, are needed to replace dopamine.
Tannin is a type of plant polyphenol that extensively exists in plant tissues and food, particularly in the roots, stems, and leaves of some plants and different beverages such as wine, tea, and cider.31 The structure of tannin, which contains abundant catechol and pyrogallol functional groups, is extremely complex which endows tannin a series of unique chemical characteristics and physiological activities.32 Recently, tannin-based surface coatings have drawn immense interest because of the outstanding surface binding affinity of tannin.33–35 Phillip B. Messersmith's group found that tannin can be coated on a variety of organic and inorganic substrates in buffered saline (pH 7.8) under the presence of oxygen, which is similar to the coating conditions of dopamine polymerization.23,31 Zhang et al. reported a novel hydrophilization method via the co-deposition of tannin and diethylenetriamine on three types of hydrophobic membrane surfaces and found that their hydrophilicity was dramatically improved.36 Compared with dopamine, the use of tannin has many advantages for surface modification, because it is colorless, rich in resources, easy to obtain and most importantly, low in cost, which indicate potential applications for tannin in PVDF surface modification.
Tara, which is obtained from the fruits pods of the Tara tree, is widely planted in South America, was reported to contain high amounts of tannin (40–60%).37 Herein, we propose the deposition of Tara tannin onto the PVDF microfiltration membrane surface via a simple dip-coating method. The plant tannin coating, which can endow PVDF porous membranes excellent hydrophilicity and outstanding filtration performance, was detected using a series of characterization techniques. We studied the influence of deposition time on surface morphology, chemical composition and wettability. It is found that a long deposition time can increase the coating content and hydrophilicity. Moreover, the modified membranes exhibit novel hydrophilic stability, which was tested by long-term water rinsing. This simple and effective strategy has wide application prospects due to its low cost and eco-friendly applications.
2. Experimental
2.1. Materials
Commercial PVDF microfiltration membranes were purchased from Membrane Solutions (Chain). Tara powder, which is made in Peru, was bought from the Shanghai CelChem Company. Isopropyl alcohol, toluene, chloroform, dichloromethane, Tween-80, sodium hydroxide and tris (hydroxymethyl)-aminomethane (Tris) were all purchased from the Sinopharm Chemical Reagent Co., Ltd (China). All chemicals were used as received. Deionized water was obtained from a deionized water generator system (RS-10B, XINRUI, Chain).
2.2. Extraction of tannin solution
A simple aqueous dissolution method was used to extract tannin. A certain amount of Tara powder mixed with deionized water (1/10, w/w) was rapidly stirred with a magnetic stirrer at room temperature for 4 h first, and then, the mixture solution was left to stand overnight for extraction. Finally, the solution was filtered through a Büchner funnel 3 times to obtain a brown tannin solution.
2.3. PVDF membrane tannin coating process
The detailed protocol for the tannin coating process is presented in Scheme 1. Commercial PVDF membranes were initially immersed in isopropyl alcohol for 0.5 h to clean the membrane surfaces and wet their pore walls and then the membranes were placed in deionized water to exchange the isopropyl alcohol in the membrane pores for at least 1 h in preparation for the modification. The extracted tannin solution was used to prepare alkaline tannin solution with Tris-buffer (100 mL, pH = 7.8, 50 mM). The pre-treated PVDF membranes were immersed in the alkaline tannin solution for a certain time (6 h, 12 h and 24 h) using open vessels for continuous contact with the atmosphere at room temperature. During this time, tannin oxidation induced aggregation occurred and tannin was deposited on the membrane surfaces to change their wettability. Then, the modified membranes were rinsed thoroughly with deionized water and placed in deionized water or dried in air for further research. The various modified membranes were named PTA-6, PTA-12 and PTA-24 according to their immersion time. For comparison, the pristine membrane was immersed in deionized water for 24 h which was named PTA-0.
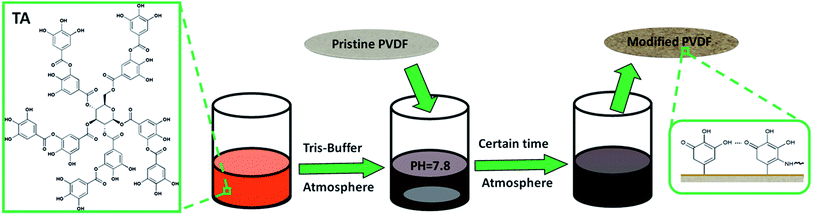 |
| | Scheme 1 Illustration of the process of tannin deposition onto PVDF membranes. | |
2.4. Tannin solution characterization
Tannin is oxidized continuously upon contact with the atmosphere in alkaline conditions, which results in a change in solution color. The chemical reaction occurring in the tannin solution with a certain time was measured using an ultraviolet spectrophotometer (UV-Vis, UV-2550, Shimadzu, Japan).
2.5. Membrane characterization
2.5.1. Morphology and chemical composition. The membrane surface morphology and microstructure were observed on a field-emission scanning electron microscope (FESEM, S4800, Hitachi, Japan) with an accelerating voltage of 5.0 kV, and all samples were sputtered with gold before the FESEM measurement. The chemical composition of the modified and unmodified membranes was analyzed via X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi, ThermoFisher, Britain) with Al-Kα as the radiation source. The functional groups on the membrane surface were determined via attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR, Nicolet 6700, ThermoFisher, USA).
2.5.2. Deposition ratio. The deposition ratio of the modified membranes was calculated using eqn (1), which employs a simple weighing method:| |
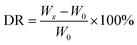 | (1) |
where DR refers to the deposition ratio and Wx and W0 are the weight of the modified and pristine membranes, respectively.
2.5.3. Contact angle. The water contact angle (WCA) and underwater oil contact angle (OCA) of the membrane surface were measured using a contact angle test system (JC2000C, Zhongchen, China), and at least five measurements were taken at different positions on each sample.
2.5.4. Water uptake content. Water uptake content can intuitively reflect the hydrophilicity of different membranes. The modified and unmodified membranes were immersed in deionized water for 24 h to reach adsorption equilibrium, then, the wetting membranes were taken out and the free water on the membrane surface was removed with filter paper. The water uptake content was calculated using eqn (2):| |
 | (2) |
where A represents the water uptake content, and Ww and Wd are the weight of the wet and dry membranes, respectively.
2.5.5. Filtration performance. The filtration performance of pure water and a toluene-in-water emulsion was measured on a vacuum driven filtration system at a stable pressure (−0.0975 MPa). The oil-in-water emulsion was prepared by mixing toluene and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) with the addition of 0.02 mg Tween-80 per milliliter of emulsion under a high stirring speed for 5 h.15 The flux recovery ratio (FRR) was used to analyze the antifouling property of the membranes, where, a higher FRR indicates a better membrane fouling resistance property. The flux and FRR values of the various membranes were calculated using eqn (3) and (4):
99, v/v) with the addition of 0.02 mg Tween-80 per milliliter of emulsion under a high stirring speed for 5 h.15 The flux recovery ratio (FRR) was used to analyze the antifouling property of the membranes, where, a higher FRR indicates a better membrane fouling resistance property. The flux and FRR values of the various membranes were calculated using eqn (3) and (4):| |
 | (3) |
| |
 | (4) |
where J is the flux value (L m−2 h−1), including pure water flux (Jw) and oil/water emulsion filtration flux (Je). V, A, and Δt represent the volume of permeated liquid, membrane area and permeation time, respectively. Jw1 and Jw2 refer to the pure water flux before and after oil/water emulsion filtration, respectively.
2.5.6. Long-term rinsing experiment. The hydrophilic stability of the modified membrane was tested via a long-term rinsing experiment. The modified membrane (PTA-12) was immobilized on the wall of a beaker that was filled with water and then the rinsing test was undertaken using a magnetic stirrer with a continuously high stirring speed for about a week. The pure water flux and underwater OCA were measured once a day to assess the stability of the modified membrane.
3. Results and discussion
3.1. Tannin solution characterization
The probable reaction occurring during the deposition time in the tannin solutions was detected via UV-Vis spectra, as shown in Fig. 1. The various measured tannin solutions were diluted 1000 times. The maximum absorbance at λ = 273 nm was caused by catechol groups in Tara tannin, and a broadening absorption peak at around λ = 325 nm appears in the alkaline tannin solutions due to the formation of quinone. The intensities of both peaks decrease with an increase in deposition time, whereas the increase in precipitation in shown in the inset image. This indicates that an oxidation induced aggregation mechanism occurred in the alkaline tannin solutions.
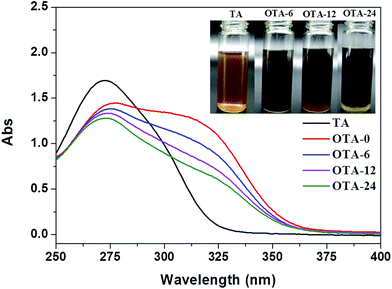 |
| | Fig. 1 UV-Vis spectra of the pristine tannin solution and alkaline tannin solution after the stipulated deposition time. Inset images are digital photos of the tannin and oxidized tannin solutions. | |
3.2. Morphology of PVDF membranes
The images of the various membranes are shown in Fig. 2. A slight change in color occurred on the modified membranes in contrast to the dark color of the PDA modified membranes in other reports.38–40 The surface morphology of the modified membranes with different immersion times and the pristine membrane was observed via FESEM, which is shown in Fig. 3a–d. As indicated by the FESEM images, the surface porosity decreases with an increase in modification time. Moreover, it can be found that a coating layer is obviously formed on the modified membrane surfaces compared with the pristine PVDF membrane. The formed coating layer can significantly enhance the wettability of PVDF membranes and improve their water treatment properties.
 |
| | Fig. 2 Images of the pristine and modified PVDF membranes. | |
 |
| | Fig. 3 FESEM images of the different PVDF membranes: (a) pristine membrane, (b) PTA-6, (c) PTA-12 and (d) PTA-24. | |
3.3. Chemical composition of PVDF membranes
The deposition ratio (DR), which is calculated using the weight of the membranes, can directly reflect the amount of tannin deposited on the membranes. The change in DR is shown in Fig. 4, where it can be observed that the DR value increases with deposition time. The DR of the membrane deposited at 24 h (PTA-24) reaches 8.08%, which indicates that more tannin can coat the membrane surface with a longer deposition time.
 |
| | Fig. 4 Deposition ratio on the PVDF membranes. | |
The chemical composition of the membranes was measured via XPS and is shown in Fig. 5 and Table 1. The small amount of O and N detected for PTA-0 may be derived from some additives for the formation of porous membranes such as a pore-forming agent. As shown in Fig. 5, for the modified membranes, the signals for F decrease dramatically compared with the pristine membrane, whereas the peak intensities of O and C immensely increase. This indicates that tannin, which contains abundant O and C elements, had been deposited on the membrane surfaces, thus resulting in a coating layer. Moreover, the quantities of the different elements on the membrane top surface are shown in Table 1, wherein it can be found that the O/C ratio increases with an extension in modification time, as well as the content of both O and C elements, which corresponds with the DR result that more tannin was coated on the membrane surface with an increase in modification time. However, the content of N element increased slightly with the modification time rather than decreased, and this can be rationalized by the reaction between the amine groups of tris and oxidized tannin through Michael addition. The O/C ratio of PTA-24 is 0.619, which is consistent with the theoretical value of tannin coating reported by others,23 thus implying the successful immobilization of the tannin coating. As indicated in Fig. S1 in the ESI,† the absorption peak at around 1608 cm−1 and some weak peaks at 1570–1500 cm−1, which are caused by the skeletal vibration of aromatic rings, are detected by ATR-FTIR in the modified membranes compared with the pristine membrane, which imply that a detectable amount of plant tannin coating was fixed on the membrane surfaces successfully.
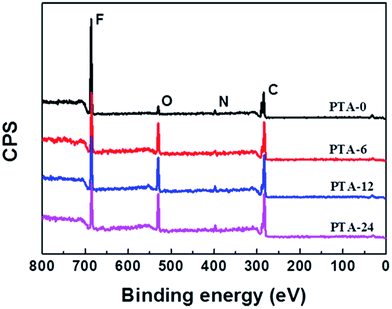 |
| | Fig. 5 XPS spectra of the pristine and modified PVDF membranes. | |
Table 1 Elemental composition of various the PVDF membranes detected by XPS
| Membrane |
Composition (%) |
O/C |
| C |
F |
O |
N |
| PTA-0 |
24.07 |
63.78 |
3.57 |
8.58 |
0.148 |
| PTA-6 |
30.86 |
43.90 |
15.71 |
9.53 |
0.509 |
| PTA-12 |
33.08 |
39.58 |
17.85 |
9.49 |
0.540 |
| PTA-24 |
33.19 |
35.55 |
20.53 |
10.73 |
0.619 |
3.4. Hydrophilicity of PVDF membranes
The hydrophilicity of the modified and unmodified membranes was measured using the water contact angle (WCA), water uptake content and underwater oil contact angle (OCA). As shown in Fig. 6a, the WCA of all the modified membranes are obviously much smaller than the pristine membrane (PTA-0), and the WCA of the modified membranes decrease with the increase in modification time. Water drop time also demonstrates the wettability of the modified membranes, as shown in Fig. 6a and b. For PTA-0, the WCA decreased slightly within 12 s and remained constant at 78° finally, which implies the poor wettability of the pristine membrane. However, for PTA-6, PTA-12 and PTA-24, the WCA decreased rapidly and all finally changed to 0° within 6 s, 5 s and 4 s, respectively, which exhibit much higher hydrophilicity than the pristine membrane. The water uptake content of the different membranes are shown in Fig. S2,† wherein it can be found that the water uptake content of the modified membranes improves from 40% to approximately 73% compared with the pristine membrane, and this also confirms the success of the hydrophilic modification of the PVDF membranes.
 |
| | Fig. 6 (a) Water contact angle and images of a water droplet on the different PVDF membranes and (b) water drop age on the different PVDF membranes. | |
The underwater OCA (chloroform) values are shown in Fig. 7a, in which all the modified PVDF membranes possess excellent oleophobicity, as indicated by the underwater OCA values of 150°, 156° and 160°, which all are higher than the pristine membrane. Different oil droplets on the modified membrane (PTA-12) surface show high underwater OCA values (all over 150°), as indicated in Fig. 7b, which exhibit the excellent oleophobicity of the membranes. Excellent underwater oleophobicity implies good hydrophilicity for the modified PVDF membranes.
 |
| | Fig. 7 (a) Underwater oil (chloroform) contact angle of the different PVDF membranes; (b) underwater OCA of different oil droplets on the modified membrane (PTA-12) surface. | |
3.5. Filtration performance of PVDF membranes
The pure water flux, emulsion flux and flux recovery ratio (FRR) were tested on a vacuum driven filtration system to evaluate filtration performance, as shown in Fig. 8. The pure water flux is shown in Fig. 8a, wherein it can be observed that the dried modified membranes have a higher flux than the pristine membrane, and the flux value of PTA-12 even reaches about 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1, which is much higher than the 8200 L m−2 h−1 of the pristine membrane. The reason for the low water flux of the pristine membrane is its inherent strong hydrophobicity which results in compaction under high pressure, thus the water flux of the wetted membranes was measured to avoid the compaction effect. It can be observed that the water flux of the wetted pristine membrane has a great improvement; however, it is still less than the modified membranes. This demonstrates that the modified membranes possess an excellent filtration performance.
000 L m−2 h−1, which is much higher than the 8200 L m−2 h−1 of the pristine membrane. The reason for the low water flux of the pristine membrane is its inherent strong hydrophobicity which results in compaction under high pressure, thus the water flux of the wetted membranes was measured to avoid the compaction effect. It can be observed that the water flux of the wetted pristine membrane has a great improvement; however, it is still less than the modified membranes. This demonstrates that the modified membranes possess an excellent filtration performance.
 |
| | Fig. 8 Filtration performance of the different membranes: (a) pure water flux of the dried and wetted PVDF membranes, (b) flux of toluene-in-water emulsions, inset image is the emulsion before (left) and after (right) filtration, and (c) flux recovery ratio after the emulsion separation. | |
The emulsion filtration performance is shown in Fig. 8b, where the emulsion separation efficiency greatly improved for the modified membranes due to their high underwater oleophobicity, and the emulsion is transformed into a transparent liquid after filtration, as shown in the inset image. It is worth noting that both the pure water and emulsion filtration flux decrease for PTA-24 compared with PTA-12, and this can be explained by the fact that their pores are blocked by tannin aggregation, as shown in the FESEM image (Fig. 2d). After emulsion filtration for 0.5 h, the pure water flux was tested to evaluate the antifouling property of the membranes, which is different from the permeability. The flux recovery ratio shown in Fig. 8c increases with an increase in modification time and the FRR of the modified membranes (PTA-6, PTA-12, and PTA-24) are all more than 95%, and even reaches 100% for PTA-24. These indicate the great antifouling and oil rejection properties of the modified PVDF membranes.
3.6. Hydrophilic stability of the modified membrane
The hydrophilic stability of the modified membrane is very important for water treatment with a high efficiency in long-term operations. Therefore, a rinsing test was carried out and both pure water flux and underwater oil (chloroform) contact angle were measured to evaluate the stability of the tannin coated PVDF membrane (PTA-12). As shown in Fig. 9, the pure water flux does not change much within a week rinsing. The underwater oil contact angle also stabilized at around 156° during the long rinsing process. These disclose the outstanding durability of the tannin coated PVDF membrane.
 |
| | Fig. 9 Pure water flux and underwater oil contact angle of PTA-12 during the long-term rinsing test. | |
4. Conclusions
In summary, a hydrophilization modified PVDF membrane is developed by depositing a plant tannin coating onto the membrane surface. By tuning the modifying time, the amount of tannin coating that affects the hydrophilicity and filtration performance is researched. Hydrophilicity improved with an increase in modification time, and the minimum water contact angle value for the tannin coated PVDF membranes even decreased to 33° (PTA-24), which is lower than the dopamine modified PVDF membranes reported by others (around 40–60°) under the same modification time.28,41 Filtration performance was also enhanced after the coating with tannin. The pure water flux increased from 8200 L m−2 h−1 for the pristine PVDF membrane to the maximum 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 (PTA-12), and the emulsion filtration flux also increased from 782 L m−2 h−1 to the maximum 2341 L m−2 h−1 (PTA-12). The flux recovery ratio of all the modified membranes is more than 95%, and PTA-24 even reaches 100%, which is higher than the 82% of the pristine PVDF membrane. Moreover, the tannin coated PVDF membranes also obtained excellent stability, as indicated by the long-term rinsing experiment. Although the interaction between the tannin coating and PVDF membrane surface is still not clear, the performance of the tannin coated PVDF membrane indicates that tannin is a promising material in the field of surface hydrophilization modification.
000 L m−2 h−1 (PTA-12), and the emulsion filtration flux also increased from 782 L m−2 h−1 to the maximum 2341 L m−2 h−1 (PTA-12). The flux recovery ratio of all the modified membranes is more than 95%, and PTA-24 even reaches 100%, which is higher than the 82% of the pristine PVDF membrane. Moreover, the tannin coated PVDF membranes also obtained excellent stability, as indicated by the long-term rinsing experiment. Although the interaction between the tannin coating and PVDF membrane surface is still not clear, the performance of the tannin coated PVDF membrane indicates that tannin is a promising material in the field of surface hydrophilization modification.
Acknowledgements
This study was supported by Program of Young Scholar sponsored by China Scholarship Council (201306955022).
References
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS PubMed.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- G. Kwon, A. K. Kota, Y. Li, A. Sohani, J. M. Mabry and A. Tuteja, Adv. Mater., 2012, 24, 3666–3671 CrossRef CAS PubMed.
- Y. Zhu, D. Wang, L. Jiang and J. Jin, NPG Asia Mater., 2014, 6, e101 CrossRef CAS.
- P. H. Duong, T. S. Chung, S. Wei and L. Irish, Environ. Sci. Technol., 2014, 48, 4537–4545 CrossRef CAS PubMed.
- Y. El Rayess, C. Albasi, P. Bacchin, P. Taillandier, J. Raynal, M. Mietton-Peuchot and A. Devatine, J. Membr. Sci., 2011, 382, 1–19 CrossRef CAS.
- G.-d. Kang and Y.-m. Cao, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS.
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- Z. Cui, E. Drioli and Y. M. Lee, Prog. Polym. Sci., 2014, 39, 164–198 CrossRef CAS.
- J. Ji, F. Liu, N. A. Hashim, M. R. M. Abed and K. Li, React. Funct. Polym., 2015, 86, 134–153 CrossRef CAS.
- M. Hossein Razzaghi, A. Safekordi, M. Tavakolmoghadam, F. Rekabdar and M. Hemmati, J. Membr. Sci., 2014, 470, 547–557 CrossRef CAS.
- T. Rajasekhar, M. Trinadh, P. Veera Babu, A. V. S. Sainath and A. V. R. Reddy, J. Membr. Sci., 2015, 481, 82–93 CrossRef CAS.
- J. Zhang, Z. Xu, W. Mai, C. Min, B. Zhou, M. Shan, Y. Li, C. Yang, Z. Wang and X. Qian, J. Mater. Chem. A, 2013, 1, 3101–3111 CAS.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed. Engl., 2014, 53, 856–860 CrossRef CAS PubMed.
- A. Qin, X. Li, X. Zhao, D. Liu and C. He, ACS Appl. Mater. Interfaces, 2015, 7, 8427–8436 CAS.
- S. Liang, Y. Kang, A. Tiraferri, E. P. Giannelis, X. Huang and M. Elimelech, ACS Appl. Mater. Interfaces, 2013, 5, 6694–6703 CAS.
- Z. Yu, Y. Pan, Y. He, G. Zeng, H. Shi and H. Di, RSC Adv., 2015, 5, 51364–51370 RSC.
- R.-S. Juang, C. Huang, H.-Y. Jheng, C. Li, L.-Y. Wu and Y.-J. Chang, J. Taiwan Inst. Chem. Eng., 2015, 54, 76–82 CrossRef CAS.
- R. F. Susanti, Y. S. Han, J. Kim, Y. H. Lee and R. G. Carbonell, J. Membr. Sci., 2013, 440, 88–97 CrossRef CAS.
- J. R. Du, S. Peldszus, P. M. Huck and X. Feng, Water Res., 2009, 43, 4559–4568 CrossRef CAS PubMed.
- J. Sedo, J. Saiz-Poseu, F. Busque and D. Ruiz-Molina, Adv. Mater., 2013, 25, 653–701 CrossRef CAS PubMed.
- D. G. Barrett, T. S. Sileika and P. B. Messersmith, Chem. Commun., 2014, 50, 7265–7268 RSC.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- H.-C. Yang, J. Luo, Y. Lv, P. Shen and Z.-K. Xu, J. Membr. Sci., 2015, 483, 42–59 CrossRef CAS.
- B. D. McCloskey, H. B. Park, H. Ju, B. W. Rowe, D. J. Miller and B. D. Freeman, J. Membr. Sci., 2012, 413–414, 82–90 CrossRef CAS.
- Y. Xiang, F. Liu and L. Xue, J. Membr. Sci., 2015, 476, 321–329 CrossRef CAS.
- B. P. Lee, P. B. Messersmith, J. N. Israelachvili and J. H. Waite, Annu. Rev. Mater. Res., 2011, 41, 99–132 CrossRef CAS PubMed.
- M. Kohri, H. Kohma, Y. Shinoda, M. Yamauchi, S. Yagai, T. Kojima, T. Taniguchi and K. Kishikawa, Polym. Chem., 2013, 4, 2696–2702 RSC.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. Lau and P. B. Messersmith, Angew. Chem., Int. Ed. Engl., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- C. Poncet-Legrand, B. Cabane, A.-B. n. Bautista-Ortín, S. Carrillo, H. l. n. Fulcrand, J. Pérez and A. Vernhet, Biomacromolecules, 2010, 11, 2376–2386 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- S. Kim, T. Gim and S. M. Kang, ACS Appl. Mater. Interfaces, 2015, 7, 6412–6416 CAS.
- L. Pan, H. Wang, C. Wu, C. Liao and L. Li, ACS Appl. Mater. Interfaces, 2015, 7, 16003–16010 CAS.
- X. Zhang, P.-F. Ren, H.-C. Yang, L.-S. Wan and Z.-K. Xu, Appl. Surf. Sci., 2016, 360, 291–297 CrossRef CAS.
- N. Bellotti, B. del Amo and R. Romagnoli, Prog. Org. Coat., 2012, 74, 411–417 CrossRef CAS.
- Z.-X. Wang, C.-H. Lau, N.-Q. Zhang, Y.-P. Bai and L. Shao, J. Mater. Chem. A, 2015, 3, 2650–2657 CAS.
- Z. Wang, X. Jiang, X. Cheng, C. H. Lau and L. Shao, ACS Appl. Mater. Interfaces, 2015, 7, 9534–9545 CAS.
- H.-C. Yang, K.-J. Liao, H. Huang, Q.-Y. Wu, L.-S. Wan and Z.-K. Xu, J. Mater. Chem. A, 2014, 2, 10225 CAS.
- J. Jiang, L. Zhu, L. Zhu, B. Zhu and Y. Xu, Langmuir, 2011, 27, 14180–14187 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13634f |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 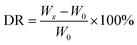

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) with the addition of 0.02 mg Tween-80 per milliliter of emulsion under a high stirring speed for 5 h.15 The flux recovery ratio (FRR) was used to analyze the antifouling property of the membranes, where, a higher FRR indicates a better membrane fouling resistance property. The flux and FRR values of the various membranes were calculated using eqn (3) and (4):
99, v/v) with the addition of 0.02 mg Tween-80 per milliliter of emulsion under a high stirring speed for 5 h.15 The flux recovery ratio (FRR) was used to analyze the antifouling property of the membranes, where, a higher FRR indicates a better membrane fouling resistance property. The flux and FRR values of the various membranes were calculated using eqn (3) and (4):





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1, which is much higher than the 8200 L m−2 h−1 of the pristine membrane. The reason for the low water flux of the pristine membrane is its inherent strong hydrophobicity which results in compaction under high pressure, thus the water flux of the wetted membranes was measured to avoid the compaction effect. It can be observed that the water flux of the wetted pristine membrane has a great improvement; however, it is still less than the modified membranes. This demonstrates that the modified membranes possess an excellent filtration performance.
000 L m−2 h−1, which is much higher than the 8200 L m−2 h−1 of the pristine membrane. The reason for the low water flux of the pristine membrane is its inherent strong hydrophobicity which results in compaction under high pressure, thus the water flux of the wetted membranes was measured to avoid the compaction effect. It can be observed that the water flux of the wetted pristine membrane has a great improvement; however, it is still less than the modified membranes. This demonstrates that the modified membranes possess an excellent filtration performance.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 (PTA-12), and the emulsion filtration flux also increased from 782 L m−2 h−1 to the maximum 2341 L m−2 h−1 (PTA-12). The flux recovery ratio of all the modified membranes is more than 95%, and PTA-24 even reaches 100%, which is higher than the 82% of the pristine PVDF membrane. Moreover, the tannin coated PVDF membranes also obtained excellent stability, as indicated by the long-term rinsing experiment. Although the interaction between the tannin coating and PVDF membrane surface is still not clear, the performance of the tannin coated PVDF membrane indicates that tannin is a promising material in the field of surface hydrophilization modification.
000 L m−2 h−1 (PTA-12), and the emulsion filtration flux also increased from 782 L m−2 h−1 to the maximum 2341 L m−2 h−1 (PTA-12). The flux recovery ratio of all the modified membranes is more than 95%, and PTA-24 even reaches 100%, which is higher than the 82% of the pristine PVDF membrane. Moreover, the tannin coated PVDF membranes also obtained excellent stability, as indicated by the long-term rinsing experiment. Although the interaction between the tannin coating and PVDF membrane surface is still not clear, the performance of the tannin coated PVDF membrane indicates that tannin is a promising material in the field of surface hydrophilization modification.





