Osteometrics in burned human skeletal remains by neutron and optical vibrational spectroscopy
Abstract
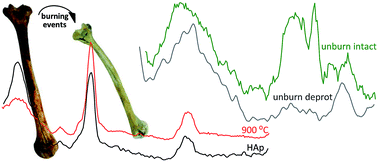
Vibrational spectroscopic techniques are applied to the osteometric study of burned human skeletal remains. This is an innovative way of tackling heat-induced changes in human bone tissue, aimed at relating burned to pre-burned parameters which will have a significant impact in forensic, bioanthropological and archaeological contexts.


 Please wait while we load your content...
Please wait while we load your content...