Enhanced upconversion luminescence and tuned red-to-green emission ratio of LiGdF4 nanocrystals via Ca2+ doping†
Zhenmin Xiong‡
,
Yushi Yang‡ and
Youfa Wang*
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Biomedical Materials and Engineering Center, Wuhan University of Technology, Wuhan 430070, China. E-mail: wangyoufa@whut.edu.cn
First published on 18th July 2016
Abstract
LiGdF4 nanoparticles (NPs) with different Ca2+ contents were synthesized by a thermal decomposition method. The as-prepared NPs were able to emit green and strong red light when excited by a 980 nm laser through a two-photon process and they were characterized by X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), and photoluminescence (PL) spectroscopy. Rietveld refinement was performed to explore the structure and phase composition of the product. It was found that Ca2+ ions were vital to the successful synthesis of LiGdF4 NPs and an appropriate Ca2+ content (ca. 19%) yielded the product with highest purity. The purity of the obtained product was highly correlated with the PL intensity and red/green light intensity ratio.
Introduction
In recent years, lanthanide (Ln)-doped upconversion nano-particles (UCNPs) have attracted considerable attention due to their wide applications in electronic devices, optical devices, biomedical devices, and solar cells.1–6 Compared with traditional fluorescence imaging agents, namely quantum dots and organic dyes, Ln-doped UCNPs possess unique advantages including improved tissue penetration depth, low radiation damage, and weak auto-fluorescence.7 The novel performances of UCNPs come from their ability to absorb near-infrared (NIR) irradiation and to emit ultraviolet or visible light.8–14However, human tissues strongly absorb the light whose wavelength is below 600 nm, a spectral range contains the light emitted by most UCNPs. Such absorption therefore limits the application UCNPs as in vivo imaging luminescent probes.15–17 One way to make an improvement is to enlarge the wavelength of the emission from UCNPs since human tissues have weaker adsorption in terms of the light whose wavelength falls into the NIR region (700–1100 nm) and red region (600–700 nm).18,19 Such spectral range were referred as “optical window” in many publications and tuning both the excitation and emission peaks of the UCNPs into the “optical window” is important for deep tissue imaging in vivo.20,21
On the other hand, among the various types of UCNPs, tetragonal LiGdF4 is an outstanding host for its high visible quantum efficiency.22 However, the syntheses of LiGdF4 via wet chemical methods was difficult23–26 and an orthorhombic GdF3 always formed as impurity prior to LiGdF4.27 In order to synthesis pure LiGdF4 nano crystal, doping was applied. For instance, Ho Seong Jang et al. successfully synthesized tetragonal phase Li(Gd,Y)F4:Yb,Er nanocrystals by doping Y3+ ions.26 The obtained UCNPs based on LiGdF4 showed stronger green up-converting (UC) luminescence in comparison with β-NaYF4 host. In addition, doping is not only a convenient method to alter the phase of the product, but also an important method to adjust the UC luminescence of UCNPs. The impact of Ca2+, Sr2+ ions introduced to UCNPs had been studied and it was found these ions were capable of breaking the symmetry of the crystal field around the rare-earth ions, leading to the enhancement of PL intensity.28–31
Herein, UCNPs with strong red UC luminescence were synthesized to meet the criteria for tissue imaging. Specifically, LiGdF4 UCNPs doped with Ca2+, Yb3+, Er3+, and Tm3+ ions were obtained by thermal decomposition method. The as-prepared NPs were characterized by X-ray diffractometer (XRD), high-resolution transmission electron microscopy (HRTEM), and photoluminescence (PL) spectrometer. Rietveld refinement was performed to explore the structure and phase fraction of the product. The obtained product was a mixture of LiGdF4, orthorhombic GdF3 and fluorite-type Gd0.67F2. The last two phases were impurities. They significantly decreased the UC emission intensity and the R/G emission ratio of LiGdF4 UCNPs. It was found that Ca2+ ions stabilized the crystal structure of LiGdF4 and a proper proportion of Ca2+ ions significantly decreased the amount of the impurity, therefore enhanced the PL performance of the product.
Experimental
Materials
Gadolinium(III) oxide (Gd2O3, 99%), ytterbium(III) oxide (Yb2O3, 99%), erbium(III) oxide (Er2O3, 99%), thulium(III) oxide (Tm2O3, 99%), 1-octadecene (ODE, 90%), oleic acid (OA, 90%), and trifuoroacetic acid (CF3COOH, 99.0%) were purchases from Aladdin Incorporation. Lithium hydroxide (LiOH, 90%), calcium hydroxide (Ca(OH)2, 95%), cyclohexane (C6H12, 97%) were purchases from Sinopharm Chemical Reagent Incorporation. Silicon (SRM™ 640e) was obtained from National Institute of Standards and Technology (NIST). All chemicals were of analytical grade and were used without further purification. Lanthanide trifluoroacetates ((CF3COO)3Ln) were prepared by dissolving the respective lanthanide oxides in trifuoroacetic acid (CF3COOH) into a 50 mL three-neck flask on a heating mantle. Lithium trifluoroacetate (CF3COOLi), calcium trifluoro-acetate ((CF3COO)2Ca) were prepared by dissolving the respective lithium hydroxide and calcium hydroxide in tri-fluoroacetic acid (CF3COOH) into a 50 mL three-neck flask on a heating mantle.Synthesis of Ca2+-doped LiGdF4:20Yb3+/4Er3+/2Tm3+ NPs
CF3COOLi (1 mmol), (CF3COO)3Gd (0.72 mmol), (CF3COO)3Yb (0.19 mmol), (CF3COO)3Tm (0.02 mmol), (CF3COO)3Er (0.04 mmol) and (CF3COO)2Ca (x = 0, 3.8, 11.4, 19.0, 26.6, 34.2, 45.6, 57.0) were added to a 50 mL flask containing OA (6 mL) and ODE (4 mL). Another 8 mL of OA was prepared in another flask. Both flasks were heated at 150 °C for 30 min under vacuum to remove water. The OA was then heated to a certain temperature 320 °C at a rate of 20 K min−1 under dry nitrogen atmosphere. The rare-earth trifluoroacetate solution at 150 °C was then injected into the OA solution after 5 minutes. After the injection, the mixture was heated to 330 °C under nitrogen atmosphere for 1 h. The solution was then cooled down to room temperature and cyclohexane was added to precipitated the NPs. The precipitate was collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and washed alternately with cyclohexane and ethanol. The obtained NPs were then dried in air at 60 °C for 4 h.
000 rpm, 5 min) and washed alternately with cyclohexane and ethanol. The obtained NPs were then dried in air at 60 °C for 4 h.
Characterization
The X-ray powder diffraction patterns were measured by a high resolution diffractometer equipped with CuKα radiation (Bruker Discover D8, λ = 1.5418 Å). Quantitative phase analysis of the XRD result was performed based on Rietveld refinement.32 The Rietveld refinement was performed with the assistance of GSASII (latest build).33 The Silicon (SRM™ 640e) was used to obtain the instrumental broadening profile. The morphology and microstructure of as-synthesized UCNPs were observed using high resolution transmission electron microscope (HRTEM, JEM-2100F STEM/EDS, JEOL Corp, Japan). The UC emission spectra were recorded using a fluorescence spectrometer (970CRT, Shanghai Sanco, China) equipped with an adjustable laser diode (980 nm, Hi-Tech Optoelectronics Co., Ltd.) as the excitation sources.Results and discussion
Fig. 1 shows the X-ray diffraction (XRD) patterns of the obtained LiGdF4:xCa2+/20Yb3+/4Er3+/2Tm3+ UCNPs (S0, x = 0; S1, x = 11.4; S2, x = 19.0; S3, x = 26.6; S4, x = 34.2; S5, x = 57.0). The diffraction peaks of S0 was weak, therefore indicates that well crystallized tetragonal LiGdF4 (JCPDS card no. 27-1236) could only be obtained with the assistance of Ca2+. It is also noticeable that a small peak appeared at about 28° for sample S1–S5, and such peak was considered belonging to the impurity phase. In the XRD patterns of sample S0 and S1, a tiny peak was observed at about 25° and it was also regarded as impurity.The result of the quantitative phase analysis of the UCNPs was shown in Fig. 2. The impurity phases were identified to be an orthorhombic GdF3, which was easy to form according previous study,27 and fluorite-type Gd0.67F2 in which all original Ca atoms were replaced by Gd atoms.34 More details on the Rietveld refinement and the corresponding fitting results were listed in the ESI (Fig. S1–S6, Tables S1–S6†). It is clear that all samples had impurities and sample S2 had the largest percentage of LiGdF4. Specifically, sample S0 had by far the least amount of LiGdF4 and this suggests that doping Ca2+ could not only increase the crystallinity of the product, but also increase the weight percentage of LiGdF4.
Sample S2, whose Ca2+ fraction was 19%, had the minimum amount of impurity phase, containing only 7.7 wt% Gd0.67F2. Sample S1 and S3 had similar phase compositions and there was GdF3 in these two samples. The phase fractions of Gd0.67F2 in sample S4, and S5 were significantly higher than that of sample S1 to S3. This might relate to the excessive Ca2+ ions added into the reaction system.
Fig. 3 shows the TEM images of sample S2. As shown in Fig. 3a, LiGdF4 NPs with uniform octahedron shapes were observed and their average size measured from the image was ca. 150 nm. Small amount of fluorite-type Gd0.67F2 NPs were also observed with much smaller sizes. The HRTEM image of the LiGdF4 was shown in Fig. 3b and the fringes corresponding to (101) plans of LiGdF4 were observed. Fig. 3c illustrates the HRTEM image of Gd0.67F2 in sample S2 and the insert shows the (111) planes of Gd0.67F2. Sample S1, S3, S4, S5 had similar morphologies as shown in the ESI (Fig. S7†).
To study the influence of Ca2+ concentration on UC emission property, sample S1–S5 were characterized by a PL spectrometer under the excitation of 980 nm laser diode. Fig. 4a shows the emission spectra of sample S1 to S5. It is clear that the spectra of these UCNPs had similar shapes but different intensities. The order of the samples according to the emission intensity was S2 > S3 > S1 > S4 > S5, being roughly identical with the order of the phase fraction of LiGdF4. Fig. 4b illustrates the deconvolution of the PL spectrum of sample S2. The spectrum was fitted with Gaussian functions. The non-linear fit was performed with the assistance of software Fityk (1.3.0).35 Six peaks appeared in the spectrum, including two main red peaks (663 and 677 nm, 4F9/2 → 2I15/2 of Er3+), two shoulder red peaks (692 and 711 nm, 3F3 → 3H6 of Tm3+) and two green peaks (535 nm, 2H11/2 → 2I15/2 of Er3+; 558 nm, 4S3/2 → 2I15/2 of Er3+).17,26,27,36
To further investigate effect of purity of the UCNPs on the UC emitting intensity, the correlation of PL emission intensity and the weight percentage of LiGdF4 of different samples were studied. Fig. 5 illustrates the relationships between intensity of PL emission peaks and the weight percentage of LiGdF4 from sample S1 to S5. The intensities were obtained by integrating corresponding peaks in the PL spectra and the weight percentages of LiGdF4 were obtained from the Rietveld refinement (Fig. 2). It is clear that all of the PL features, namely the intensity of red peaks (600 to 750 nm), the intensity of green peaks (500 to 600 nm) and the intensity ratio between red and green peaks, had linear relationships with the weight percentage of LiGdF4. This indicates that higher purity of LiGdF4 UCNPs yields higher UC luminescence intensity and a larger red component in the emitted light.
 | ||
| Fig. 5 Relationships between intensities of PL emission peaks and the weight percentage of LiGdF4 from sample S1 to S5. (a) Relationship between the integration intensity of red emission peaks (650 to 750 nm) and the LiGdF4 weight percentage; (b) relationship between the integration intensity of green emission peaks (500 to 600 nm) and the LiGdF4 weight percentage; (c) relationship between the red/green intensity ratio and the LiGdF4 weight percentage. The five scatters came from the five samples (S1 to S5), with the sample name labels nearby. The fitting parameters and equations are available in the ESI (Table S7†). | ||
In order to determine the number of photons responsible for the UC emission, the luminescence intensities were measured as a function of the pump power for sample S2. For the unsaturated UC process, the number of photons required to populate the upper emitting state can be described by the following relation:37,38
| Iup ∝ INIRn |
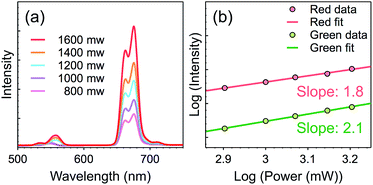 | ||
| Fig. 6 Pump power dependence of the UC emissions in sample S2. (a) The spectra of sample S2 excited by 980 nm laser with different power; (b) the log–log plots of UC emission intensity versus pumping density. The fitting parameters and equations are available in the ESI (Table S7†). | ||
Conclusions
LiGdF4 NPs doped with Ca2+, Yb3+, Er3+ and Tm3+ were synthesized by thermal decomposition method. The obtained NPs were able to emit green and strong red light when excited by 980 nm laser through a two-photon process. It was found that Ca2+ ions were required to synthesis the LiGdF4 NPs and a proper amount of Ca2+ (ca. 19%) yielded the product with highest purity. The purity of the obtained product was highly correlated with both the PL intensity and the red/green light intensity ratio. The strong red UC emission of the obtained NPs implies they could be applied for tissue imaging.Acknowledgements
This work was financially supported by National College Students' innovation and entrepreneurship training of China (20151049701024).Notes and references
- J.-C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847–852 CrossRef CAS PubMed.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937–943 CrossRef CAS PubMed.
- G. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. K. Pandey, H. Ågren, P. N. Prasad and G. Han, ACS Nano, 2012, 6, 8280–8287 CrossRef CAS PubMed.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- W. Zheng, P. Huang, D. Tu, E. Ma, H. Zhu and X. Chen, Chem. Soc. Rev., 2015, 44, 1379–1415 RSC.
- M. He, X. Pang, X. Liu, B. Jiang, Y. He, H. Snaith and Z. Lin, Angew. Chem., Int. Ed. Engl., 2016, 55, 4280–4284 CrossRef CAS PubMed.
- L. Cheng, C. Wang and Z. Liu, Nanoscale, 2013, 5, 23–37 RSC.
- G. Dantelle, M. Mortier, G. Patriarche and D. Vivien, J. Solid State Chem., 2006, 179, 1995–2003 CrossRef CAS.
- N.-N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramírez-Hernández, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. a. Capobianco, J. G. Solé, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed.
- Y.-P. Du, Y.-W. Zhang, L.-D. Sun and C.-H. Yan, Dalton Trans., 2009, 8574–8581 RSC.
- Q. Huang, J. Yu, E. Ma and K. Lin, J. Phys. Chem. C, 2010, 114, 4719–4724 CAS.
- K. Konig, J. Microsc., 2000, 200, 83–104 CrossRef CAS PubMed.
- L. Lei, D. Chen, P. Huang, J. Xu, R. Zhang and Y. Wang, Nanoscale, 2013, 5, 11298–11305 RSC.
- L. Lei, D. Chen, J. Xu, R. Zhang and Y. Wang, Chem.–Asian J., 2014, 9, 728–733 CrossRef CAS PubMed.
- C. Li, J. Liu, S. Alonso, F. Li and Y. Zhang, Nanoscale, 2012, 4, 6065–6071 RSC.
- M. Misiak, A. Bednarkiewicz and W. Stręk, J. Lumin., 2016, 169 Part B, 717–721 CrossRef.
- D. Yang, G. Li, X. Kang, Z. Cheng, P. Ma, C. Peng, H. Lian, C. Li and J. Lin, Nanoscale, 2012, 4, 3450–3459 RSC.
- N. Niu, P. Yang, F. He, X. Zhang, S. Gai, C. Li and J. Lin, J. Mater. Chem., 2012, 22, 10889 RSC.
- G. Tian, Z. Gu, L. Zhou, W. Yin, X. Liu, L. Yan, S. Jin, W. Ren, G. Xing, S. Li and Y. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- F. Vetrone, J. C. Boyer, J. A. Capobianco, A. Speghini and M. Bettinelli, J. Appl. Phys., 2004, 96, 661 CrossRef CAS.
- C. Wang, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 1110–1120 CrossRef CAS PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- J. Wang, F. Wang, C. Wang, Z. Liu and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 10369–10372 CrossRef CAS PubMed.
- R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Science, 1999, 283, 663–666 CrossRef CAS PubMed.
- H. Na, J. S. Jeong, H. J. Chang, H. Y. Kim, K. Woo, K. Lim, K. A. Mkhoyan and H. S. Jang, Nanoscale, 2014, 6, 7461–7468 RSC.
- M. Yang, Y. Sui, S. Wang, X. Wang, Y. Sheng, Z. Zhang, T. Lü and W. Liu, Chem. Phys. Lett., 2010, 492, 40–43 CrossRef CAS.
- W. Yin, L. Zhao, L. Zhou, Z. Gu, X. Liu, G. Tian, S. Jin, L. Yan, W. Ren, G. Xing and Y. Zhao, Chemistry, 2012, 18, 9239–9245 CrossRef CAS PubMed.
- X.-F. Yu, L.-D. Chen, M. Li, M.-Y. Xie, L. Zhou, Y. Li and Q.-Q. Wang, Adv. Mater., 2008, 20, 4118–4123 CrossRef CAS.
- J.-H. Zeng, J. Su, Z. Li, R.-X. Yan and Y.-D. Li, Adv. Mater., 2005, 17, 2119–2123 CrossRef CAS.
- S. Zeng, G. Ren, C. Xu and Q. Yang, CrystEngComm, 2011, 13, 4276–4281 RSC.
- D. L. Bish and S. a. Howard, J. Appl. Crystallogr., 1988, 21, 86–91 CrossRef CAS.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- D. Steele, P. E. Childs and B. E. F. Fender, J. Phys. C: Solid State Phys., 1972, 5, 2677 CrossRef CAS.
- S. Zeng, G. Ren and Q. Yang, J. Mater. Chem., 2010, 20, 2152 RSC.
- M. Wojdyr, J. Appl. Crystallogr., 2010, 43, 1126–1128 CrossRef CAS.
- D. Zhou, P. Zhou, D. Liu, W. Xu, Y. Zhu, S. Xu, Q. Dai and H. Song, Opt. Lett., 2014, 39, 4619–4622 CrossRef CAS PubMed.
- J. Zhou, Z. Liu and F. Li, Chem. Soc. Rev., 2012, 41, 1323–1349 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Rietveld refinement results and some TEM images of the product. See DOI: 10.1039/c6ra13441f |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |




