Paris saponin I inhibits proliferation and promotes apoptosis through down-regulating AKT activity in human non-small-cell lung cancer cells and inhibiting ERK expression in human small-cell lung cancer cells†
Zhen Liua,
Qi Zhenga,
Wenzhu Chena,
Shuli Mana,
Yuou Tenga,
Xin Menga,
Yongmin Zhangb,
Peng Yu*a and
Wenyuan Gao*c
aChina International Science and Technology Cooperation Base of Food Nutrition/Safety and Medicinal Chemistry, Key Laboratory of Industrial Fermentation Microbiology of Ministry of Education, Tianjin Key Laboratory of Industry Microbiology, College of Biotechnology, Tianjin University of Science & Technology, Tianjin 300457, P. R. China. E-mail: yupeng@tust.edu.cn
bUniversité Pierre et Marie Curie-Paris 6, Institut Parisien de Chimie Moléculaire UMR CNRS 8232, 4 place Jussieu, 75005, Paris, France
cTianjin Key Laboratory for Modern Drug Delivery & High-Efficiency, School of Pharmaceutical Science and Technology, Tianjin University, Tianjin 300072, China. E-mail: pharmgao@tju.edu.cn
First published on 20th July 2016
Abstract
Paris Saponin I (PSI), a steroidal saponin derivative extracted from a traditional Chinese herbal Paris polyphylla, has shown cytotoxic effects on several tumor cell lines. However, the mechanisms of its antitumor activity especially for lung cancers remain to be elucidated. In this present investigation, we continue to explore the efficacy and mechanisms underlying the cytotoxic effects of PSI in lung cancer cell lines. Three non-small cell lung cancer (NSCLC) cells (H1299, H520, H460) and one small cell lung cancer (SCLC) cell (H446) were treated with PSI for the first time. PSI significantly induced cell cycle arrest at the G2/M phase and mitochondrial-related apoptosis NSCLC cells but not SCLC cells. Additionally, PSI reduced phosphorylation of AKT in NSCLC and ERK in SCLC in general. Interestingly, we observed that PSI influenced different signaling pathways among the four kinds of lung cancer cells. After PSI treatment, p38 MAPK and ERK activation were observed in H1299, while p38 MAPK increased and JNK decreased in H520. On the contrary, we found JNK activation in H460 cells with PSI. However, PSI upregulated the AKT activity and inhibited the JNK expression in H446 cells. The results indicate that PSI exhibits the cytotoxicity in different pathways depending on the cancer types.
1. Introduction
Lung cancer remains the most frequent cancer worldwide and is the leading cause of cancer death in men in 87 countries and women in 26 countries. There are four major histological types of lung cancer: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell lung cancer (SCLC), the former three are belonged to non-small-cell lung cancers (NSCLC).1 About 80% of lung cancer patients are of NSCLC type and the 5 year survival across all stages is about 12%.2 Several target drugs such as erlotinib, gefitinib, afatinib and crizotinib were approved by FDA and achieved a better outcome for advanced NSCLC patients. However, these drugs are limitation and show major therapeutic for the mutation of the epidermal growth factor receptor (EGFR) NSCLC cases.3 Thus, new drugs or strategies are still required for wide type EGFR (wt EGFR) NSCLC and SCLC patients.The uncontrolled proliferation of cancer cells is usually associated with alteration of growth factor receptors such as the EFGR family in mutation lung cancer. This is often accompanied with the activation of various intracellular signal pathways causing enhancing cellular proliferation and/or decreased apoptosis.4–6 Two downstream signaling pathways including Ras-Mitogen Activated Protein Kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)-AKT are activated by EGFR in mutation cancer cells, resulting in uncontrolled growth and cell proliferation. The classical MAPK pathway consists of the extracellular-signal-regulated kinase (ERK), the c-jun N-terminal kinase or stress-activated protein kinase (JNK); and p38 MAPK. Once activated, MAPK exerts an important role in converting extracellular stimuli into a wide range of cellular responses, including proliferation, differentiation, senescence and so on.7 However, emerging research additionally reported that the signaling pathways were influenced the outcome after drug treatment in wt EGFR mutation cancer cells.8–10 As reported, Ras-MAPK pathway inhibition may block a number of pro-metastatic mechanisms in SCLC cells. Thus, RAF inhibitors may be particularly useful in patients with localized disease or with few metastases that could be removed surgically, to help delay the appearance of additional metastatic lesions.11
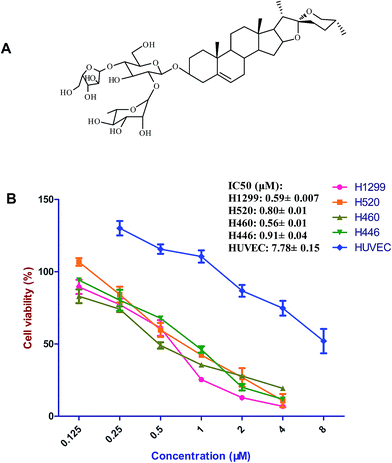
Paris Saponin I (PSI), also called polyphyllin D (Fig. 1A) is a potent cytotoxic steroidal saponin isolated from Paris polyphylla. Paris polyphylla as a traditional Chinese herbal has been applied to treat diseases in the past decade. Several saponins were obtained from this plant and have been declared to exhibit numerous pharmacological effects.1,12,13 Among the saponins, PSI has been demonstrated to exert a wide range of pharmacological activities and cytotoxicity against multiple human cancer cell lines by inducing apoptosis or cell cycle arrest in human breast cancer cells,14 HepG2 liver cancer cells,15 U87 human glioma cells,16 and SKOV3 human ovarian cancer cells.17 It was also reported that PSI affected the human lung cancer cells such as A549, SK-MES-1 and H460 or the resistant lung cancer cells by downregulating B-cell lymphoma 2 (Bcl-2), upregulating caspase-3, Bcl-2-like protein 4 (Bax) and arresting cell cycle.18–21 For the signaling pathways, PSI elicited the apoptosis in SKOV3 cells U87 human glioma cells by activation of ERK and JNK.12,16 Nevertheless, molecular mechanism involved in PSI-mediated cytotoxicity is still needed for understanding its anticancer properties especially for lung cancer. In this study, we investigated the antitumor effect of PSI on four wt EGFR lung cancer cells including human lung adenocarcinoma NCI-H1299 (H1299), human lung squamous cell carcinoma NCI-H520 (H520), human lung large cell carcinoma NCI-H460 (H460) and SCLC NCI-H446 (H446) cells for the first time. Meanwhile, the mechanisms of PSI association with Ras-MAPK and PI3K-AKT signaling pathways involved in cell cycle arrest and cell apoptosis were investigated.
2. Materials and methods
2.1 Drugs and reagents
PSI was purchased from Dalian Meilun Biology Technology Co. Ltd. (Liaoning, China) with purity > 98%.2.2 Cell lines and culture
Human NSCLC cell lines (H1299, H520 and H460) and SCLC line (H446) were kindly donated by Prof. Zhi Yao (Tianjin Medical University, Tianjin, China). Human umbilical vein endothelial cells (HUVEC) were used to test the toxicity of PSI on normal cells. All of them were purchased from the China Center for Type Culture Collection (Shanghai, China). All cell lines were cultured in RPMI 1640 medium with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin–streptomycin (100 units per mL, 100 μg mL−1) at 37 °C in a humidified incubator containing 5% CO2.2.3 Cell viability measured by MTT assay
Cell viability was evaluated using a MTT assay. Growing cells (5 × 104/mL) were seeded in 96-well plates and treated on the following day with increasing concentrations (0.125–4 μM or 0.25–8 μM) of PSI for 48 h. The cells were co-incubated with DMSO alone as a control. MTT solution (5 mg mL−1) was added to each well, the plates were incubated for additional 4 h at 37 °C and then formazan crystal were dissolved in DMSO. The cell viability was determined by measuring the absorbance at 490 nm against the reference wavelength of 630 nm using an automated microplate spectrophotometer. Half of the inhibition concentration (IC50) was the test drug concentration that caused 50% growth inhibition, which were calculated by GraphPad Prism 5 software. Each concentration/assay was performed in triplicate.2.4 Cell cycle determined by flow cytometry
Cell cycle distribution was assessed using propidium iodide (PI) staining. Lung cancer cells were seeded in 6-well plates (2 × 105 cells) and incubated for 24 h to allow attachment. After exposure to DMSO and PSI (2 and 4 μM) for 12, 24 and 48 h, both of the adherent and floating cells were harvested and washed with phosphate buffered saline (PBS), and then re-suspended in 75% ice-cold ethanol for fixing and stored at −20 °C for 24 h. The fixed cells were washed twice with PBS, and the cell pellets were then incubated in a buffer contain 50 μg mL−1 PI, 0.2% Triton-X-100, and 100 μg mL−1 RNase A. After 20 min in the dark at room temperature, the four phases of sub-G1, G1, S and G2/M analysis was performed by BD Accuri C6 FACScan flow cytometer (USA). Integration of the area under the curve for each of the phases of the histogram was performed with ModFit LT software.2.5 Quantification of apoptosis by Annexin V/propidium iodide staining
Apoptosis in lung cancer cells was induced with 2 and 4 μM PSI for 48 h. Apoptotic cells were determined using an Annexin V-FITC Apoptosis Detection Kit (Tianjin Sungene Biotech Co., Ltd.). All the operations were performed according to the user manual. Briefly, the four lung cancer cells were seeded in 6-well plates at a density of 2 × 105 cells per well. After treatment with 2 and 4 μM PSI for 48 h, the cells were harvested and washed twice with PBS, and then stained with Annexin V-FITC/PI according to the manufacturer's procedure. The samples were analyzed with a BD FACSCanto II flow cytometer (Becton Dickinson).2.6 Measurement of mitochondrial membrane potential (MMP)
The loss of MMP was evaluated utilizing tetramethyl rhodamine methyl ester (TMRM) fluorescent dye in the lung cancer cells. The four lung cancer cells were seeded in 6-well plates and treatment with 2 and 4 μM PSI for 48 h. The cells were harvested and washed twice with PBS and stained with 100 nM TMRM for 30 min at 37 °C in the dark. Subsequently the stained cells were assayed by flow cytometry. Results were expressed as the proportion of cells with low TMRM fluorescence indicating the loss of ΔΨm.2.7 Caspase assay
The lung cancer cells (H1299, H520, H460 and H446) were treated by PSI (2 and 4 μM) for 12 h to determine cellular apoptosis, which was detected using the Apoone homogeneous caspase 3/7 (protease activity) assay (Promega, In Vitro Technologies, Madison, WI, USA) and performed according to the manufacturer's instructions. Caspase 3/7 activity in the cell lysate was measured at an excitation wavelength of 485 nm and an emission of 521 nm on a Synergy 4 Multi-mode microplate reader (BioTek).2.8 Western blot analysis
Cells were homogenized in chilled lysis buffer comprising 10 mM Tris–HCl (pH 7.4), containing 1% NP-40, 0.1% deoxycholic acid, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, and 1% protease inhibitor cocktail (Sigma, Tokyo, Japan) and stood for 30 min on ice. After centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, the supernatants were collected as protein samples.
000 rpm for 10 min at 4 °C, the supernatants were collected as protein samples.
Protein contents were measured using a BSA protein assay. The cell extract was diluted by 6× SB loading buffer (Sigma-Aldrich, St. Louis, MO) and boiled for 5 min before loading onto SDS-PAGE gels. The protein samples were separated by SDS-PAGE using a 10% or 12% polyacrylamide gel and electro-blotted onto a PVDF membrane. After blockage of non-specific binding sites for 1 h with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBST), the membrane was incubated overnight at 4 °C with various primary antibodies. They included anti-PARP (BD Biosciences, USA), anti-caspase-8 and -9 (Cell Signaling Technology Inc.), anti-AKT1, anti-ERK1/2, anti-phospho-AKT1 (Tianjin Sungene Biotechnology, Tianjin), anti-JNK, anti-p38 MAPK, anti-phospho-ERK1/2, anti-phospho-JNK, anti-phospho-p38 MAPK (Santa Cruz Biotechnology, Santa Cruz, CA). An anti-tublin antibody (Sigma-Aldrich) was used as a control for equal loading. After washed 3 times with PBST, the membranes were incubated further with HRP-conjugated sheep anti-mouse or anti-rabbit IgG antibody (Cell Signaling Technology Inc.) at room temperature for 1 h at room temperature, and then washed 3 times with PBST. The immunoblots were visualized by Odyssey infrared imaging system (LI-COR Biotechnology, USA). Relative band densities of targets, determined densitometrically and normalized to that of tublin were analyzed using Image-pro plus 6.0 software.
2.9 Statistical analysis
Data were expressed as mean ± S.D. or percentage and analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Dunnett's test. P-Value less than or equal to 0.05 was considered to be statistically significant. Statistical analyses were carried out in GraphPad Prism 5 (GraphPad Software, San Diego, CA, U.S.A.).3. Results
3.1 Effects of PSI on the cell viability of the lung cancer cell lines
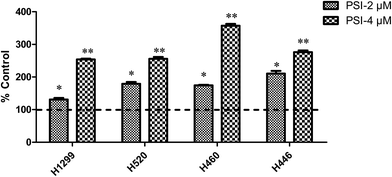
The cell viability lines of the lung cancer cells showed concentration-dependent manner after being treated with PSI (0.125–4 μM or 0.25–8 μM). PSI significantly inhibited the proliferation of the four lung cancer cell lines. Three NSCLC cell lines were more sensitive to PSI than H446 belonged to SCLC. In order to test the selective of PSI, we chose HUVEC as the normal cells and found PSI showed much less sensitive to the normal cells than that of lung cancer cells (Fig. 1).3.2 Cell cycle distribution after treatment of PSI
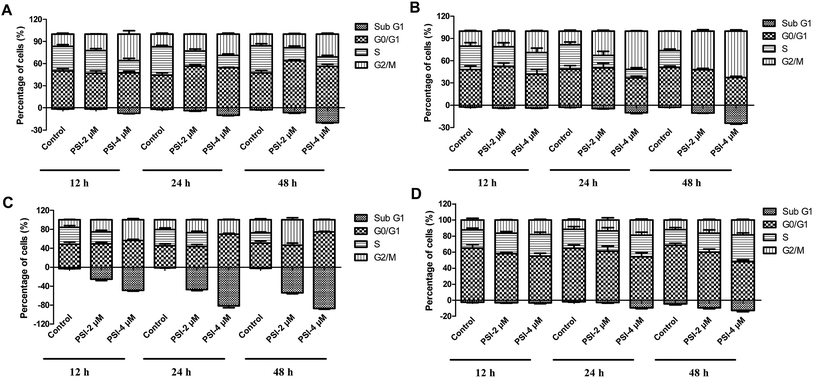
The effects of PSI on cell cycle distribution were evaluated in the four lung cancer cells using PI staining and flow cytometry analysis. The results revealed that the cell cycle progression in four lung cancer cell lines was disrupted in different ways after 12, 24, and 48 h of PSI treatment (Fig. 2 and S1†). PSI treatment caused a concentration-dependent increase of G2/M phase cell population in H1299 cells within 48 h, indicating PSI was able to arrest the cell cycle in G2/M phase (Fig. 2A). In addition, PSI caused a concentration and time-dependent accumulation of cells at the sub G1 phase. PSI treatment also induced a G2/M arrest and sub G1 accumulation in H520 cells. However, PSI may affect cell cycle progression to mitosis (G2/M transition)22 in H520 cells (Fig. 2B). When treated H460 cells, PSI gave a dramatic increase in the number of cells in the sub G1 phase in addition to the growth of the number cells in the G2/M phase (Fig. 2C). Unlike other three lung cancer cells, H446 cells were slightly induced a S phase accumulation as well as the sub G1 phase with concentrations of PSI (Fig. 2D). Collectively, the present results suggest that PSI inhibits cancer cell growth at least partly through induction of cell apoptosis (sub G1 phase) and cell cycle arrest in G2/M phase in H1299, H520 and H460 cells.3.3 Cell apoptosis induced by PSI
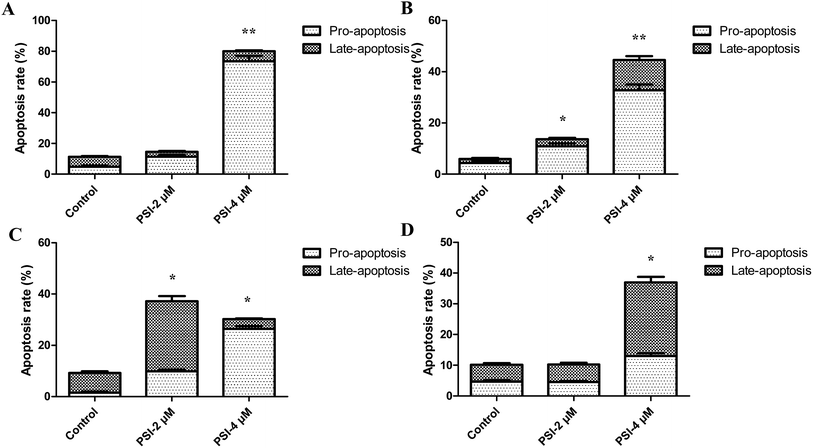
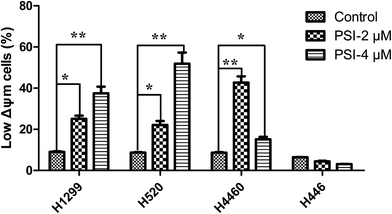
Sub G1 phase represented the apoptosis and necrosis. To investigate whether PSI induced lung cancer cells inhibition associated with cell apoptosis, we examined the effect of PSI on apoptotic cell death by flow cytometry following Annexin V-FITC and PI staining. PSI at 4 μM dramatically induced early-stage apoptotic cells when treated H1299 and H520 cells, especially in H1299 cells, which reached to 73.54 ± 3.44% (Fig. 3A and B). For H460 cell, PSI remarkably increased the late-stage apoptotic cells at 2 μM, while improved early-stage apoptotic cells at 4 μM (Fig. 3C). As to the H446 cells, PSI affected late-stage apoptotic cells at 4 μM (Fig. 3D).After treating lung cancer cells with PSI, the state of mitochondrial membranes of the cells was estimated by TMRM. The control cells showed high fluorescence due to accumulation of the fluorescent dye within the inner membrane of intact mitochondria. The cells incubated with increasing concentrations of PSI demonstrated a decrease in fluorescence. Results indicated that PSI cause loss of MMP in the H1299, H520 and H460 cells but not H446 cells (Fig. 4 and S3†). Additionally, cells exposed to PSI treatment showed dose-dependent loss of MMP in both of H1299 and H520 cells. The obtained results show that mitochondria play a vital role in induction of apoptosis in the NSCLC (H1299, H520, and H460 cells) treated with PSI.
To further confirm the contribution of apoptosis induction to PSI mediated cell death, we determined the caspase activity using the Apo-one® homogeneous caspase-3/7 assays. A dose-dependent apoptosis effect of PSI was observed and a significant increase in caspase 3/7 activity compared to controls. The four lung cancer cell lines were relatively sensitive to PSI exhibiting a 2–4 folds increase in caspase activity at 4 μM (Fig. 5).
3.4 The levels of apoptosis-related proteins after PSI treatment
To further confirm the contribution of apoptosis induction to PSI-mediated cell death, immunoblot analyses against PARP and caspase 8, 9 were performed on the four lung cancer cell lines treated with PSI. The presence of cleaved PARP, which ultimately leads to tumor cell apoptosis, was remarked increased in the presence of PSI in H1299 cells and H520 cells compared with the controls (Fig. 6A and B), although the results were not observed in H460 cells and H446 cells (Fig. 6C and D). Caspase 8 and 9 mediate the extrinsic (death ligand) and intrinsic (mitochondrial) apoptotic pathways, respectively. Results illustrated in Fig. 6 show that PSI treatment gave increased expression of cleaved caspase 9 in H1299 cells, H520 cells and H460 cells, and decreased expression of caspase 8 and caspase 9 compared to control group.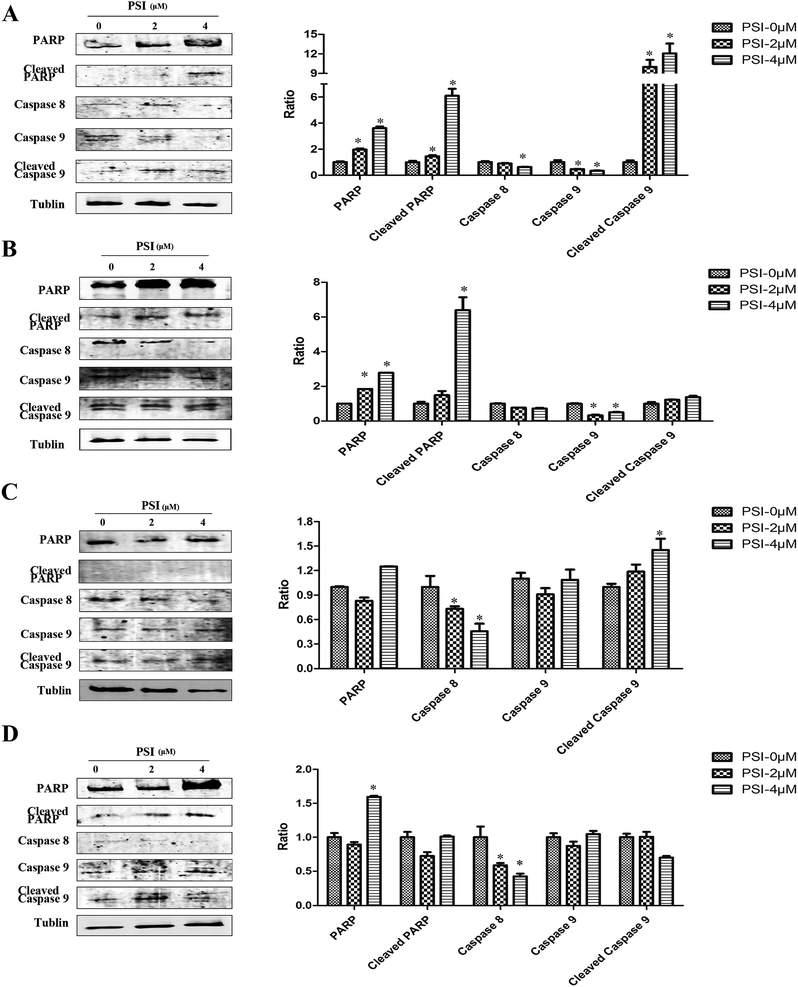 | ||
| Fig. 6 Western blots of cells treated with PSI at the concentration of 2 and 4 μM show PARP and caspase 3, caspase 8, and caspase 9 in H1299 (A), H520 (B), H460 (C) and H446 (D) cells. | ||
3.5 PSI inhibits the signaling pathways
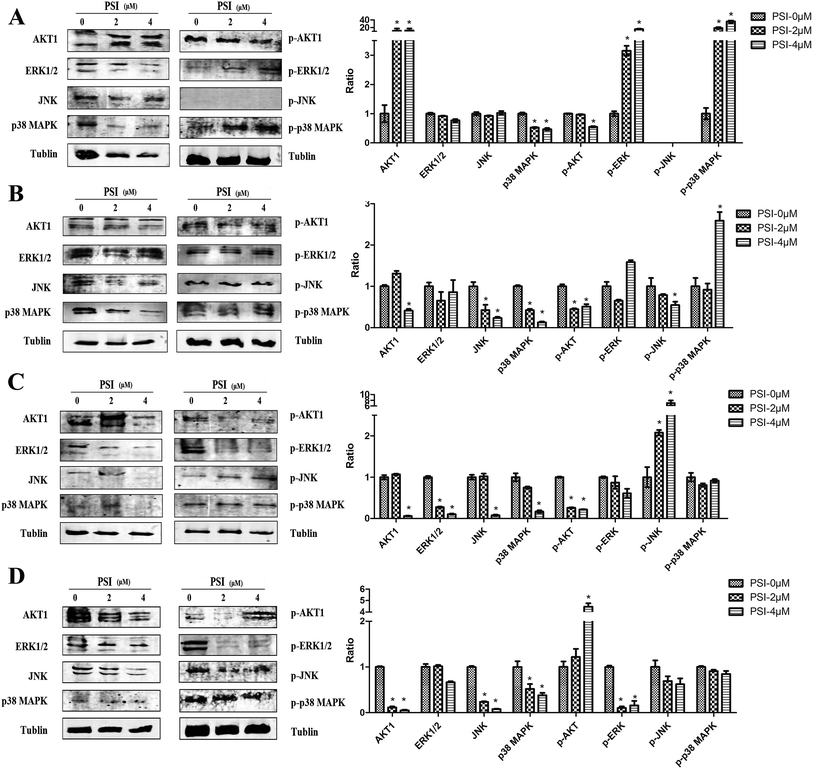
To clarify the potential involvement of various protein kinase signaling pathway responsible for the cell death in the lung cancer cells treated with PSI, we next examined the effect of PSI on AKT1, ERK, JNK, p38 MAPK and phosphorylation of these proteins activation in the lung cancer cell lines. PSI significantly upregulated AKT1 and downregulated p38 MAPK, while no significant differences were observed in ERK and JNK in H1299 cells. In the same cells, the basal levels of p-ERK and p-p38 MAPK expression were markedly higher than the control, on the contrary, p-AKT was significantly inhibited in a concentration-dependent manner after treatment with PSI (Fig. 7A). The levels of AKT1, p-AKT1, JNK, p-JNK and p38 MAPK proteins were significantly downregulated following by PSI treatment in H520 cells. However, PSI treatment did not alter the expression of ERK and p-ERK protein levels (Fig. 7B). In H460 cells, PSI significantly reduced the levels of AKT1, ERK, JNK, p 38 MAPK and p-AKT at 4 μM, while exhibited remarkable enhanced the expression of p-JNK (Fig. 7C). PSI treatment displayed a moderate decrease in ERK, p-JNK and p38 MAPK expression compared to control group, and dramatically lowered AKT1, JNK, p38 MAPK and p-ERK (Fig. 7D).4. Discussion
In the past ten years, Paris saponins including I, II, VI, and VII were investigated the antitumor properties and they can induce programmed cell death, inhibit tumor growth and metastasis.23,24 A number of studies showed that PSI could impart protection against liver cancer,25 glioma cells,16 osteosarcoma cells,12 breast cancer,14 and so on. While PSI also showed well antitumor effect on lung cancer13,18–20,26 it did not enough to define the activity for the complex of the lung cancer. In the present study, we examined the antitumor effects and the underlying mechanism of PSI on H1299, H520, and H446 cells for the first time. The signaling pathways involved in the antitumor process after PSI treatment was firstly investigated.It has been shown that the growth-inhibitory effect of PSI is attributed to apoptosis and/or cell cycle arrest, depending on the cell line studied. Our results exhibited that the apoptosis induced by PSI is accompanied by cell cycle arrest in lung cancer cell lines (Fig. 2 and S1†), which was related to caspase 3 activity (Fig. 3, 5 and S2†). During the apoptotic program, the dissipation of the inner mitochondrial transmembrane potential marks the point-of-no-return. Mitochondrial depolarization is involved in outer mitochondrial membrane permeability which is induced by many physiological effectors. Thus, evaluation of Δψm depolarization is of critical importance for the assessment of apoptosis.27 Paris saponin II, which have remarkable chemical structural similarity to PSI, induced the apoptotic mechanism in colorectal cancer involved activation of caspase-2 and the dysfunction of mitochondria.28 PSI was also reported that it triggered apoptosis in HepG2 cells through mitochondrial injury.25 Similarly, PSI elicited mitochondrial transmembrane depolarization in NSCLC, but not in SCLC (Fig. 4 and S3†).
Dysregulation of Ras-MAPK and PI3K-AKT signaling pathways play a key role in the progression of cancer cells.4,6,7,11 A number of studies reported that the signaling pathways were also regulated to achieve a better outcome by some compounds in wt EGFR cancer cells.10,29,30 Moreover, Paris saponin II and VII were reported to induce apoptosis by regulating Ras-MAPK and PI3K-AKT signaling pathways.24,31 Our major finding is that the activation of signaling pathways is critical for the induction of apoptosis in lung cancer cells exposed to PSI. PSI induced apoptosis involves p38 MAPK activation as well as AKT inhibition in H1299 and H520 cells, but JNK activation in H460 cells and ERK inhibition in H446 cells. Induction of AKT activation causes tumorigenesis and becomes a problem for treating cancer. Many studies have demonstrated that there is 50–70% overexpression of phosphorylated AKT in NSCLC indicating that abnormal activation of the PI3K-AKT pathway is a frequent event.7 Interestingly, we found the expression of AKT protein was reduced after PSI treatment in NSCLC, but activated in H446 cells (Fig. 7 and S4†). The reason for such disparate regulation of the protein activity in response to PSI is unclear, but might be related to the distinct genetic background of different types of cancer cells or the resistance induced by PSI. AKT contributes to drug resistance of cancer cells and inhibition of AKT signaling sensitized cancer cells to chemotherapy drugs.9
All of ERK, JNK and p38 MAPK belonged to MAPK family, play vital roles during cancer progression, and have been shown to be activated during the apoptotic death of tumor cells in response to various cellular stresses. The activation of the ERK pathway by growth factors often stimulates cell differentiation mitosis and hypertrophy; ERK also phosphorylates caspase-9 resulting in the inhibition of caspase-9 processing and caspase-3 activation. As reported, the reduction of ERK activation accompanied by the activation of caspase-9 and caspase-3 were elicited by PSI treatment in SKOV3 cells.17 Similarly, PSI decreased the level of ERK expression in H446 cells but upregulated p-ERK followed by inhibition of caspase 9 in H1299 cells (Fig. 7 and S4†). The unusual phenomenon given by PSI in H1299 and H446 cells could be explained as the same to the above results as the AKT expression in the cancer cells.
Recent work has suggested that MAPK family, especially p38 MAPK and JNK, play a key role in crosstalk between autophagy and apoptosis induced by genotoxic stress. There is much evidence to support a role for p38 MAPK as a tumor suppressor, and its function is mostly mediated by both negative regulation of cell cycle and apoptosis. Various chemotherapeutic agents require p38 MAPK activity for inducing apoptosis.6 p38 MAPK can be negatively regulated cell cycle progression both at G1/S and G2/M transitions by several mechanisms, including the downregulation of cyclins and upregulation of cyclin-dependent kinase (CDK) inhibitor,4 which explained that PSI arresting the G2/M phase in H1299 and H520 cells but not in H460 (Fig. 2). Pro-apoptotic function of JNK was proposed to be mediated by mitochondrial pathway. PSI significantly upregulated the expression of p-JNK associated with apoptosis in U87 human glioma cells.16 Despite the fact that mitochondrial dysfunction was observed in NSCLC, JNK activation was exhibited in H460 cells after PSI treatment. However, we could confirm that PSI induced apoptosis was not related to the JNK pathway as for without detriment to mitochondrial and the reduction JNK activity (Fig. 4 and 7).
All of AKT, ERK, JNK and p38 MAPK can mediate cell proliferation, migration and apoptosis in response to extracellular stimuli such as chemotherapeutic agents.4,5 However, crosstalk between different signaling pathways is a shared theme in cell regulation, which is usually highly dependent on cell context. Our study indicates that PI3K/AKT- and Ras/MAPK signaling pathways in the wt EGFR NSCLC and SCLC cells were distinct regulated by PSI. The detailed mechanism by which activation of the signaling pathways promote PSI-induced apoptosis needs further study. In our previous study, target drugs such as erlotinib and gefitinib showed no effect on the four lung cancer cell lines but PSI enhanced the sensitive of target drug to the cancer lines, which suggested that PSI could be as a chemo-sensitizer (data not shown). In summary, our findings provided evidences that PSI can induce apoptosis by reducing AKT pathway activation and ERK pathway activation in NSCLC and SCLC cells, respectively.
Conflict of interest
We have no conflict of interest in this research.Abbreviations
| PSI | Paris saponin I |
| NSCLC | Non-small-cell lung cancers |
| SCLC | Small cell lung cancer |
| EGFR | Epidermal growth factor receptor |
| wt EGFR | Wide type EGFR |
| MAPK | Mitogen activated protein kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| ERK | Extracellular-signal-regulated kinase |
| JNK | c-jun N-terminal kinase |
| MMP | Mitochondrial membrane potential |
| TMRM | Tetramethyl rhodamine methyl ester |
Acknowledgements
The authors thank Prof. Zhi Yao (Tianjin Medical University) for the gift of lung cancer cell lines. This project was funded by grants 2015M570229 from Postdoctoral Science Foundation of China, 2013DFA31160 from International Science & Technology Cooperation Program of China, 15JCTPJC56200 from Science and Technology Commissioner Foundation of Tianjin, and 1504A301 from Undergraduate Innovation Foundation of Tianjin University of Science & Technology.References
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- F. M. Siu, D. L. Ma, Y. W. Cheung, C. N. Lok, K. Yan, Z. Yang, M. Yang, S. Xu, B. C. Ko, Q. Y. He and C. M. Che, Proteomics, 2008, 8, 3105–3117 CrossRef CAS PubMed.
- K. Takezawa, V. Pirazzoli, M. E. Arcila, C. A. Nebhan, X. Song, E. de Stanchina, K. Ohashi, Y. Y. Janjigian, P. J. Spitzler, M. A. Melnick, G. J. Riely, M. G. Kris, V. A. Miller, M. Ladanyi, K. Politi and W. Pao, Cancer Discovery, 2012, 2, 922–933 CrossRef CAS PubMed.
- X. Sui, N. Kong, L. Ye, W. Han, J. Zhou, Q. Zhang, C. He and H. Pan, Cancer Lett., 2014, 344, 174–179 CrossRef CAS PubMed.
- O. Tetsu, J. Phuchareon, D. W. Eisele, M. J. Hangauer and F. McCormick, Pharmacol. Res., 2015, 102, 132–137 CrossRef CAS PubMed.
- E. F. Wagner and A. R. Nebreda, Nat. Rev. Cancer, 2009, 9, 537–549 CrossRef CAS PubMed.
- P. Y. Yip, Transl. Lung Cancer Res., 2015, 4, 165–176 CAS.
- S. H. Baek, J. H. Ko, J. H. Lee, C. Kim, H. Lee, D. Nam, J. Lee, S. G. Lee, W. M. Yang, J. Y. Um, G. Sethi and K. S. Ahn, J. Cell. Physiol., 2016 DOI:10.1002/jcp.25426.
- Y. Yang, T. Ikezoe, C. Nishioka, K. Bandobashi, T. Takeuchi, Y. Adachi, M. Kobayashi, S. Takeuchi, H. P. Koeffler and H. Taguchi, Br. J. Cancer, 2006, 95, 1653–1662 CrossRef CAS PubMed.
- R. S. Zhao, W. Wang, J. P. Li, C. M. Liu and L. He, Technol. Cancer Res. Treat., 2016 DOI:10.1177/1533034616643884.
- S. Cristea and J. Sage, J. Thorac. Oncol., 2016 DOI:10.1016/j.jtho.2016.04.018.
- J. Chang, H. Wang, X. Wang, Y. Zhao, D. Zhao, C. Wang, Y. Li, Z. Yang, S. Lu, Q. Zeng, J. Zimmerman, Q. Shi, Y. Wang and Y. Yang, J. Ethnopharmacol., 2015, 170, 117–127 CrossRef CAS PubMed.
- H. He, L. Zheng, Y. P. Sun, G. W. Zhang and Z. G. Yue, Asian Pac. J. Cancer Prev., 2014, 15, 10911–10916 CrossRef PubMed.
- M. S. Lee, J. C. Yuet-Wa, S. K. Kong, B. Yu, V. O. Eng-Choon, H. W. Nai-Ching, T. M. Chung-Wai and K. P. Fung, Cancer Biol. Ther., 2005, 4, 1248–1254 CrossRef CAS PubMed.
- J. Y. N. Cheung, R. C. Y. Ong, Y. K. Suen, V. Ooi, H. N. C. Wong, T. C. W. Mak, K. P. Fung, B. Yu and S. K. Kong, Cancer Lett., 2005, 217, 203–211 CrossRef PubMed.
- Q. Yu, Q. Li, P. Lu and Q. Chen, J. Med. Food, 2014, 17, 1036–1042 CrossRef CAS PubMed.
- X. Xiao, P. Bai, T. M. Bui Nguyen, J. Xiao, S. Liu, G. Yang, L. Hu, X. Chen, X. Zhang, J. Liu and H. Wang, Mol. Cancer Ther., 2009, 8, 1179–1188 CrossRef CAS PubMed.
- M. Kong, J. Fan, A. Dong, H. Cheng and R. Xu, Acta Biochim. Biophys. Sin., 2010, 42, 827–833 CrossRef CAS PubMed.
- J. Zhang, Y. Yang, L. Lei and M. Tian, Med. Sci. Monit., 2015, 21, 2535–2541 CrossRef PubMed.
- P. Zhao, H. Jiang, D. Su, J. Feng, S. Ma and X. Zhu, Mol. Med. Rep., 2015, 11, 327–332 CAS.
- H. Jiang, P. J. Zhao, D. Su, J. Feng and S. L. Ma, Mol. Med. Rep., 2014, 9, 2265–2272 CAS.
- F. Y. Long, Y. S. Chen, L. Zhang, X. Kuang, Y. Yu, L. F. Wang, X. J. Liu, L. Wang, Y. F. Zhou, N. Sang and J. R. Du, J. Ethnopharmacol., 2015, 162, 112–120 CrossRef CAS PubMed.
- Z. Lin, Y. Liu, F. Li, J. Wu, G. Zhang, Y. Wang, L. Lu and Z. Liu, Phytother. Res., 2015, 29, 1568–1576 CrossRef CAS PubMed.
- L. Zhang, S. Man, Y. Wang, J. Liu, Z. Liu, P. Yu and W. Gao, Chem.-Biol. Interact., 2016, 253, 125–133 CrossRef CAS PubMed.
- R. C. Ong, J. Lei, R. K. Lee, J. Y. Cheung, K. P. Fung, C. Lin, H. P. Ho, B. Yu, M. Li and S. K. Kong, Cancer Lett., 2008, 261, 158–164 CrossRef CAS PubMed.
- H. Jiang, P. Zhao, J. Feng, D. Su and S. Ma, Oncol. Lett., 2014, 7, 2059–2064 CAS.
- S. Jayaraman, J. Immunol. Methods, 2005, 306, 68–79 CrossRef CAS PubMed.
- J. C. Lee, C. L. Su, L. L. Chen and S. J. Won, Cancer Sci., 2009, 100, 503–513 CrossRef CAS PubMed.
- Z. X. Xia, Z. X. Li, M. Zhang, L. M. Sun, Q. F. Zhang and X. S. Qiu, Exp. Mol. Pathol., 2016, 100, 353–360 CrossRef CAS PubMed.
- J. C. Ko, H. C. Chiu, J. J. Syu, Y. J. Jian, C. Y. Chen, Y. T. Jian, Y. J. Huang, T. Y. Wo and Y. W. Lin, Biochem. Pharmacol., 2014, 88, 119–127 CrossRef CAS PubMed.
- Y. Li, Y. Sun, L. Fan, F. Zhang, J. Meng, J. Han, X. Guo, D. Zhang, R. Zhang, Z. Yue and Q. Mei, Biochem. Pharmacol., 2014, 88, 150–157 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13352e |
| This journal is © The Royal Society of Chemistry 2016 |