Thermally stable transparent sol–gel based active siloxane–oligomer materials with tunable high refractive index and dual reactive groups
Abstract
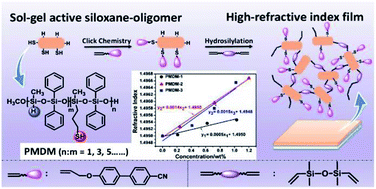
In this paper, a series of novel active silicone–oligomers (PMDM) of high refractive index (RI) with dual reactive moieties (Si–H and S–H) synthesized by nonhydrolytic sol–gel condensation are reported for the first time. The RI of the oligosiloxane can be effectively increased from 1.55 to 1.65 by varying the feed ratio between methyldimethoxysilane (MDMS) and 3-mercaptopropylmethyldimethoxysilane (MMDS). Additionally, via “thiol–ene” click chemistry and the subsequent hydrosilylation reaction under a Pt catalyst, a transparent siloxane-polymer film with enhanced refractive index (1.59) and excellent thermal stability (T5% = 381 °C) was facilely synthesized and characterized, which in turn effectively substantiated the reactivity of the dual reactive moieties in PMDM. Such active oligomers would be useful chemical intermediates and the easily processed materials have great potential in modern optical/photonic applications such as waveguides, lasers or light emitting diodes.

 Please wait while we load your content...
Please wait while we load your content...