Direct access to stabilized CuI using cuttlebone as a natural-reducing support for efficient CuAAC click reactions in water†
Abstract
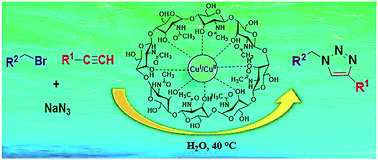
Cuttlebone@CuCl2 has efficiently catalyzed the one-pot azidonation of organic bromides, followed by a regioselective azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reaction to produce the corresponding 1,4-disubstituted 1,2,3-triazole derivatives in excellent yields, in water. Importantly, cuttlebone is not only used for successful immobilization of CuCl2 in order for its heterogenation, but it is also utilized as a natural-reducing platform which can reduce CuII to the click-active stabilized CuI. This mild three component synthetic approach avoids handling of hazardous and toxic azides, because of their in situ generation. Moreover, the catalyst has the advantages of being ligand-free, leaching-free, thermally stable, easy to synthesize from inexpensive commercially available precursors, environmentally friendly and recyclable for at least seven times without a significant decrease in its activity and selectivity.

 Please wait while we load your content...
Please wait while we load your content...