Bio-based plant oil polymers from ROMP of norbornene modified with triglyceride from crude red palm olein†
Abstract
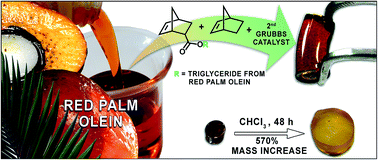
A novel monomer norbornene palm olein (NPO) was isolated from reaction of 5-norbornene-2-carboxylic acid with triglycerides from red palm olein, with one norbornene (NBE) unit per NPO. No polymer from ROMP of NPO with a 2nd generation Grubbs catalyst was produced. ROMP of NPO in the presence of free NBE at 30 °C with different amounts of each monomer resulted in products with different physical states. Viscous liquids, soft solids, and cracked solids resulted from reactions with 90%, 80–40%, and 20% NPO, respectively. The soft solids were insoluble in water, acetic acid, and typical organic solvents. Swelling experiments in slightly polar organic solvents resulted in a mass gain of up to 800%, as in chloroform at 30 °C for 48 h. SEM micrographic images showed non-porous materials, and TGA analyses indicated thermal stability up to 300 °C. A poly(NPO-co-NBE) type has been suggested as the resulting product.

 Please wait while we load your content...
Please wait while we load your content...