Synthesis of 4-thio-5-(2′′-thienyl)uridine and cytotoxicity activity against colon cancer cells in vitro†
Abstract
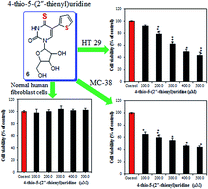
A novel anti-tumor agent 4-thio-5-(2′′-thienyl)uridine (6) was synthesized and the in vitro cytotoxicity activity against mice colon cancer cells (MC-38) and human colon cancer cells (HT-29) was evaluated by MTT assay. The results showed that the novel compound had antiproliferative activity toward MC-38 and HT-29 cells in a dose-dependent manner. The cell cycle analysis by flow cytometry indicated that compound 6 exerted in tumor cell proliferation inhibition by arresting HT-29 cells in the G2/M phase. In addition, cell death detected by propidium iodide staining showed that compound 6 efficiently induced cell apoptosis in a concentration-dependent manner. Moreover, the sensitivity of human fibroblast cells to compound 6 was far lower than that of tumor cells, suggesting the specific anti-tumor effect of 4-thio-5-(2′′-thienyl)uridine. Taken together, novel compound 6 effectively inhibits colon cancer cell proliferation, and hence would have potential value in clinical application as an antitumor agent.

 Please wait while we load your content...
Please wait while we load your content...