pHLIP-modified magnetic nanoparticles for targeting acidic diseased tissue†
A. M. Demin‡
a,
A. G. Pershina‡*bc,
K. V. Nevskayab,
L. V. Efimovab,
N. N. Shchegolevad,
M. A. Uimind,
D. K. Kuznetsove,
V. Ya. Shure,
V. P. Krasnova and
L. M. Ogorodovab
aPostovsky Institute of Organic Synthesis of RAS (Ural Branch), Yekaterinburg, Russia
bSiberian State Medical University, Tomsk, Russia. E-mail: allysyz@mail.ru; Fax: +7 382 2533309; Tel: +7 382 2901101 ext. 1634
cRussian National Research Tomsk Polytechnic University, Tomsk, Russia
dMikheev Institute of Metal Physics of RAS (Ural Branch), Yekaterinburg, Russia
eUral Centre for Shared Use “Modern Nanotechnology”, Institute of Natural Sciences, Ural Federal University, Yekaterinburg, Russia
First published on 13th June 2016
Abstract
Covalent immobilization of a pH-low insertion peptide (pHLIP) onto Fe3O4 magnetic nanoparticles was carried out resulting in the formation of MRI-visible materials able to specifically accumulate in acidic damaged tissue. The pH-dependent pHLIP-mediated binding of the obtained nanoconjugates to cells in acidic environment was demonstrated on HTC cells in vitro and in a mouse LLC tumour model in vivo.
pHLIPs (pH Low Insertion Peptides)1 are a class of peptides possessing pH-dependent transmembrane activity; these peptides are able to insert into the cell membrane at slightly acidic pH (<7.0). Acidification of the intercellular space is a property of tumours, inflammation, ischemia as well as other types of damaged tissue. Therefore, pHLIPs have been successfully used to direct therapeutic molecules2,3 and tracers4,5 toward acidic tissue.6
The purpose of this work was to develop an approach for the production of a new nanoconjugate based on Fe3O4 magnetic nanoparticles (MNPs) modified by 3-aminopropylsilane (APS) and conjugated to pHLIP, and to test it as a magnetic resonance imaging (MRI) visible contrast agent for acidic tissue (e.g. tumour) targeting.
One of the most effective methods for the modification of MNPs for biomedical applications is covalently binding organic molecules that are pre-modified by alkoxysilane reagents,7 for example, 3-aminopropyltrimethoxysilane (APTMS). To the best of our knowledge, there is a limited number of publications on the immobilization of pHLIP on the surface of inorganic nanoparticles for selective transport into acidic tissue.8,9 In particular, there is no information on obtaining MNP–pHLIP nanoconjugates. Taking into account the high potential of magnetic nanoparticles in diagnosis (e.g. MRI) as well as therapy,10 for instance magnetic hyperthermia in a high frequency magnetic field or magneto-mechanical actuation in a low frequency alternating magnetic field,11 the design of such a nanoconjugate seems to hold promise. In order to preserve their biological activity, it is generally accepted to conjugate pHLIPs at the amino group of L-Ala (N-terminal amino acid) or the thiol group of L-Cys.
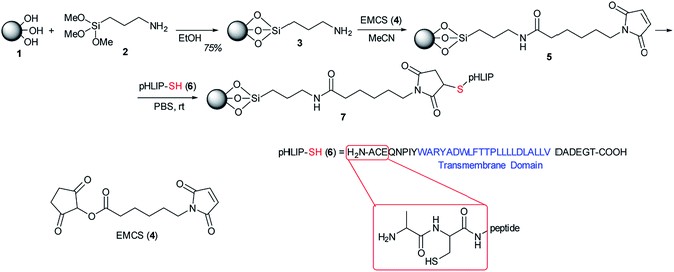
In this work a method for the immobilization of pHLIP to APS-modified MNPs (MNP-APS) through covalent binding to the L-Cys thiol group using 6-maleimidohexanoic acid N-hydroxysuccinimide ester (EMCS) as a linker is offered (Scheme 1).
Derivatives containing the maleimide moiety have been used for modification of the SH groups in biomolecules,12,13 and their conjugation to nanoparticles.14,15 The nucleophilic addition reaction of thiols to the double bond of the maleimide residue results in a stable 3-thio-succinimidyl ester. Thus, the proposed approach of covalently binding pHLIP to the MNP surface will provide the nanoconjugate with stability in the biological environments it is used in.
MNPs with an average diameter of 10 nm and spinel phase state Fe3O4 (Fig. 1A) were prepared by co-precipitation from Fe3+/Fe2+ salt solutions. In the first step, the surface of the nanoparticles (1) was modified using APTMS (2) similar to a previously described procedure16,17 (Scheme 1). The number of APS residues immobilized on the surface was 0.75 mmol g−1 according to elemental analysis based on the measurement of carbon content in the sample, as well as IR spectroscopy data18 (Fig. S1†).
Then, EMCS (4) cross-linker was attached to MNP-APS through the coupling of the hydroxysuccinimide-activated carboxyl group to the amino groups on the MNP surface followed by conjugation with pHLIP (Scheme 1).
Immobilization of pHLIP on the MNPs was confirmed by IR spectroscopy (Fig. 2). In the FTIR spectra of nanoconjugate (7) the characteristic absorption bands of the MNPs (ν 546 cm−1, Fe–O) and pHLIP (ν 3272 cm−1, stretching vibrations of NH; 2922 cm−1, stretching vibrations of CH; 1648 cm−1, amide I; 1533 cm−1, amide II; 1453 and 1390 cm−1 amide III) were indicated. Small shifts in the absorption band positions were observed due to pHLIP binding to the MNP surface (S1†). The number of pHLIP residues immobilized on the surface of the MNPs was calculated from the carbon content in the sample, similar to ref. 17 and amounted to 6.7×10−6 mol g−1 (27.6 mg g−1 MNPs) (S2†).
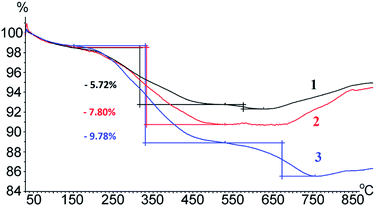
According to the thermogravimetric analysis (TGA) (Fig. 3), heating the obtained MNPs to 150 °C resulted in a weight loss (1.2%), which is associated with the thermodesorption of physically adsorbed water from the nanoparticle surface. Further heating to 530 °C led to the decomposition and removal of organic molecules bound to the surface of MNPs. The weight loss of these samples was related to the decomposition of 3-aminopropylsilane, 6-maleimidohexanoyl and peptide fragments, and through their partial removal as simple volatiles (for example, H2O, CO2, NH3) in the gaseous phase.
The comparison between values of the weight loss of MNP-EMCS and MNP-APS, MNP-pHLIP and MNP-EMCS allows us to calculate the amount of EMCS and pHLIP (20.8 and 19.8 mg g−1 MNPs correspondingly). The lower amount of a peptide on a MNP surface according to TGA data in comparison with the elemental analysis data can be explained by incomplete decomposition and removal of organic molecules fragments from the MNP surface.
The hydrodynamic characteristics of MNP-pHLIP suspensions in aqueous media were studied using dynamic light scattering. It was shown that the obtained nanoconjugate forms stable suspensions in water (mean particle hydrodynamic diameter, Dh = 155 nm; polydispersity index, PdI = 0.2; zeta potential = −22 mV), and in DMEM with 10% fetal bovine serum (Dh = 180 nm, PdI = 0.2, zeta potential = −10 mV) (S3†).
According to the transmission electron microscopy (TEM) data, significant changes in the morphology of the MNPs after surface modification and conjugation with the peptide did not occur: the average diameter was 10 nm and the nanoparticle phase state remained unchanged (Fig. 1B–D). According to scanning electron microscopy (SEM), MNP-pHLIP particles have a spherical shape with an average diameter of about 12 nm (Fig. 1E and F); (S4†). The slight difference between these data can be explained by the fact that the shell formed by a peptide had low contrast in the TEM images and was almost invisible.
The specific magnetization of MNP-pHLIP was 52 emu g−1 and was lower than the magnetization of initial MNPs (81 emu g−1), which can be explained by the presence of a silane coating and peptide on the surface of MNPs (S5†).
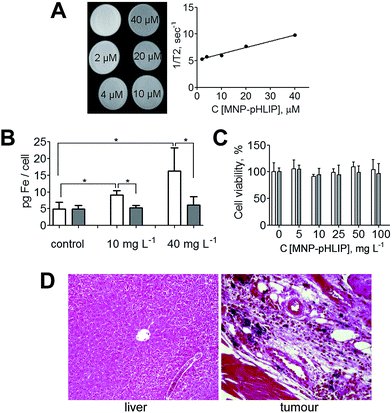
Relaxivity r2 is a key feature of MNPs used in MRI. The higher the relaxivity is, the amount of nanoconjugate that can be detected in the tissues by MRI will be lower. To characterize the MR contrast properties of MNP-pHLIP, the T2 relaxivity of nanoconjugate suspensions with different concentration were registered at 11.7 T (Fig. 4A). The calculated value of r2 was equal to 117.21 mmol−1 s−1 and exceeded that of some commercial contrast agents approved by the FDA (USA), for example, Feridex and Combidex (98 and 60 mmol−1 s−1, respectively).19
The efficiency of the MNP-pHLIP nanoconjugate binding to the cells at normal (7.4) and weakly acidic pH (6.0) was studied in vitro in the rat hepatoma (HTC) cell line8 (Fig. 4B). The cells incubated with MNP-pHLIP at pH 6.0 showed a 1.7–2.7-fold higher cell uptake in comparison with the cells incubated at pH 7.4 (according to the measurements of iron concentration in cell lysate by ferrozine-based assay20). These data evidenced the pH-dependent pHLIP-mediated uptake of the nanoconjugate by the cells.
The ability of the nanoconjugate to accumulate in acidic tissue in vivo was studied in the LLC tumour model in mice. Forty hours after the nanoconjugate was intravenously administered, an intense accumulation of nanoparticles in the tumour, though not in the liver, was observed by histological analysis (Fig. 4D). In the meantime, 40 hours after the intravenous injection of the parent (non-targeted) MNP-APS, no iron accumulation in tumour tissue was observed (S6†).
According to the MTT test,21 the obtained nanoconjugate at a concentration up to 100 mg L−1 did not exhibit cytotoxicity in the HTC cells (Fig. 4C).
Conclusions
Immobilization of pHLIP onto Fe3O4-based MNPs was carried out for the first time. pHLIP was fixed on the APS-modified MNP surface using a heterofunctional linker, EMCS, through conjugation to the peptide thiol group. It has been shown that the resulting nanoconjugate exhibits good MRI contrast properties, binds efficiently to cells in a weakly acidic environment both in vitro and in vivo and has no cytotoxic effects on the cells. Thus, the obtained pHLIP-modified MNPs can be used as a MR-visible contrast agent for tumour targeting.Acknowledgements
This work was supported by the Russian Science Foundation (project no. 14-15-00247). The authors thank Prof. Sergey V. Vtorushin for conduct the histological analysis, Dr Igor A. Klimov for helping with animal experiments, Artyom A. Minin for providing DLS analysis and Oleg B. Shevelev for assisting in MRI scanning.Notes and references
- J. C. Deacon, D. M. Engelman and F. N. Barrera, Arch. Biochem. Biophys., 2015, 565, 40 CrossRef CAS PubMed.
- M. An, D. Wijesinghe, O. A. Andreev, Y. K. Reshetnyak and D. M. Engelman, PNAS, 2010, 107, 20246 CrossRef CAS PubMed.
- C. J. Cheng, B. Bahal, I. A. Babar, Z. Pincus, F. Barrera, C. Liu, A. Svoronos, D. T. Braddock, P. M. Glazer, D. M. Engelman, W. M. Saltzman and F. J. Slack, Nature, 2015, 518, 107 CrossRef CAS PubMed.
- S. Macholl, M. S. Morrison, P. Iveson, B. E. Arbo, O. A. Andreev, Y. K. Reshetnyak, D. M. Engelman and E. Johannesen, Mol. Imag. Biol., 2012, 14, 725 CrossRef PubMed.
- A. L. Vāvere, G. B. Biddlecombe, W. M. Spees, J. R. Garbow, D. Wijesinghe, O. A. Andreev, D. M. Engelman, Y. K. Reshetnyak and J. S. Lewis, Cancer Res., 2009, 69, 4510 CrossRef PubMed.
- M. C. M. Arachchige, Y. K. Reshetnyak and O. A. Andreev, J. Biotechnol., 2015, 202, 88 CrossRef CAS PubMed.
- C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC.
- L. Yao, J. Daniels, A. Moshnikova, S. Kuznetsov, A. Ahmed, D. M. Engelman, Y. K. Reshetnyak and O. A. Andreev, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 465 CrossRef CAS PubMed.
- Z. Zhao, H. Meng, N. Wang, M. J. Donovan, T. Fu, M. You, Z. Chen, X. Zhang and W. Tan, Angew. Chem., Int. Ed., 2013, 52, 7487 CrossRef CAS PubMed.
- Y. I. Golovin, S. L. Gribanovsky, D. Y. Golovin, N. L. Klyachko, A. G. Majouga, A. M. Master, M. Sokolsky and A. V. Kabanov, J. Controlled Release, 2015, 219, 43 CrossRef CAS PubMed.
- Q. A. Pankhurst, N. K. T. Thanh, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2009, 42, 224001, DOI:10.1088/0022-3727/42/22/224001.
- J. Conde, J. T. Dias, V. Gracú, M. Moros, P. V. Baptista and J. M. de la Fuente, Front. Chem., 2014, 2, 48, DOI:10.3389/fchem.2014.00048.
- G. K Ehrlich, H. Michel, T. Truitt, W. Riboulet, P. Pop-Damkov, P. Goelzer, D. Hainzl, F. Qureshi, B. Lueckel, W. Danho, K. Conde-Knape and A. Konkar, Bioconjugate Chem., 2013, 12, 18 Search PubMed.
- I. Vikholm and W. Alberts, Langmuir, 1998, 14, 3872 CrossRef.
- I. Tsiapa, E. K. Efthimiadou, E. Fragogeorgi, G. Loudos, A. D. Varvarigou, P. Bouziotis, G. C. Kordas, D. Mihailidis, G. C. Nikiforidis, S. Xanthopoulos, D. Psimadas, M. Paravatou-Petsotas, L. Palamaris, J. D. Hazle and G. C. Kagadis, J. Colloid Interface Sci., 2014, 433, 163 CrossRef CAS PubMed.
- A. M. Demin, V. P. Krasnov and V. N. Charushin, Mendeleev Commun., 2013, 23, 14 CrossRef CAS.
- A. M. Demin, A. Yu. Vigorov, I. A. Nizova, M. A. Uimin, N. N. Shchegoleva, A. E. Ermakov, V. P. Krasnov and V. N. Charushin, Mendeleev Commun., 2014, 24, 20 CrossRef CAS.
- A. M. Demin, O. V. Koryakova and V. P. Krasnov, J. Appl. Spectrosc., 2014, 81, 565 CrossRef CAS.
- Y.-X. J. Wang, Quantitative Imaging in Medicine and Surgery, 2011, 1, 35 Search PubMed.
- J. Riemer, H. H. Hoepken, H. Czerwinska, S. R. Robinson and R. Dringen, Anal. Biochem., 2004, 331, 370 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13178f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |