Binaphthyl-based molecular barrier materials for phosphoric acid poisoning in high-temperature proton exchange membrane fuel cells†
Dong-Cheol Jeong‡
a,
Bohyun Mun‡b,
Hyekyung Leea,
Seung Jun Hwang§
c,
Sung Jong Yooc,
EunAe Chod,
Yunmi Lee*b and
Changsik Song*a
aDepartment of Chemistry, Sungkyunkwan University, 2066 Seobu-ro, Jangan-gu, Suwon, Gyeonggi 16419, Republic of Korea. E-mail: songcs@skku.edu
bDepartment of Chemistry, Kwangwoon University, 20 Kwangwoon-ro, Nowon-gu, Seoul 01897, Republic of Korea. E-mail: ymlee@kw.ac.kr
cFuel Cell Research Center, Korea Institute of Science and Technology (KIST), 5 Hwarang-ro 14-gil, Seongbuk-gu, Seoul 02792, Republic of Korea
dDepartment of Materials Science & Engineering, Korea Advanced Institute of Science and Technology, 291 Daehak-ro, Yuseong-gu, Daejeon 34141, Republic of Korea
First published on 17th June 2016
Abstract
In this study, thiol-functionalized binaphthyl barrier molecules were designed and synthesized for eliminating phosphoric acid (PA)-poisoning on Pt catalysts in oxygen reduction reactions (ORRs). In high-temperature proton exchange membrane fuel cell, the ORR activity of Pt catalysts significantly decreases because of the PA poisoning. The binaphthyl thiol (BNSH) molecules with a tweezer-like structure can self-assemble on the Pt surface, thereby blocking the adsorption of PA, while permitting the approach of smaller oxygen molecules. After the treatment of Pt surfaces with BNSHs, the ORR activities were tested in the presence of PA, and the results were compared with respect to the molecular structures of BNSHs. Even in the presence of PA, the ORR activity of BNSH-treated Pt catalysts appeared to restore significantly up to the level of the pristine Pt without PA (kinetic current density at 0.8 V from 12 to 20.4 mA cm−2). This enhanced activity was attributed to the physical blocking of PA molecules on Pt surface and was affected by the molecular structures such as tweezer backbone, length of alkyl chains, and the type and number of functional groups.
Introduction
Proton exchange membrane fuel cells (PEMFCs) have been widely studied as a green energy technology owing to their high-energy conversion efficiency, reduced pollutant emission, and other advantages.1–3 PEMFCs can be classified into low-temperature and high-temperature PEMFCs (LT- and HT-PEMFC, respectively) depending on the operating temperature.4 LT-PEMFC is limited to <100 °C, because it is basically under humidified conditions. Although significant developments have been achieved, a complex reforming system is required, because highly pure hydrogen should be introduced to prevent CO poisoning on catalysts (Pt). Moreover, a humidifier is necessary, limiting low-cost, light-weight device, and miniaturization.5,6 In contrast, the HT-PEMFC system can be simplified, because at elevated temperature, the electrochemical reactions and CO tolerance can improve, thus eliminating the need of a complex reforming system. Furthermore, a humidification system will not be required to maintain a certain level of humidity. Lastly, it can reduce hydroplaning from the flooding of the anode and reuse waste heat from high temperature.2,7 Although HT-PEMFC has many advantages, the cost per power output remains high, and the fuel-cell lifetime still needs to be improved. Polybenzimidazole (PBI)-based membranes are most commonly used as a proton exchange membrane in HT-PEMFCs,8 because acid-doped PBIs have high proton-conductivity,9 good mechanical properties10 and excellent thermal stability11 at temperatures up to 200 °C. Phosphoric acid (PA) is normally utilized in PBI-based HT-PEMFCs. The main factor of the reduced catalytic activity in HT-PEMFC is the poisoning of the catalyst by the tetrahedral anions (i.e., phosphate ions), as they tend to adsorb strongly on the Pt surface.12–14 To improve the catalytic activity of HT-PEMFC, the surface interaction of catalysts with activity-degrading anion species15 such as phosphates should be minimized.16 Cuesta, Markovic, and coworkers reported that decorating the Pt surface with cyanide (CN−) molecules through chemical coordination successfully prevented undesirable anions (SO42− and PO43−) from binding to the Pt surface, while allowing the oxygen reduction reaction (ORR) to proceed smoothly.17 Inspired by their work, we designed and synthesized thiol-functionalized binaphthyls as the molecular barrier materials for undesirable anion-bindings in ORR. We envisioned that the tweezer-like structure of binaphthyls can block the access of phosphate anions to prevent poisoning (Fig. 1), and oxygen molecules with smaller size than phosphate ions can readily pass through the binaphthyls, enabling efficient ORR. The binaphthyl thiols (BNSHs) were demonstrated to self-assemble on Pt surface, significantly enhancing the ORR activities. In addition, the effect of molecular structures on the ORR activities of various binaphthyl derivatives was investigated.Experimentals
Materials
6,6′-Dibromo-1,1′-bi-2-naphthol, tert-butyllithium (1.7 M solution in pentane), sulfur, potassium carbonate, 1-bromohexane, acetyl chloride, and Nafion® 117 solution were purchased from Sigma-Aldrich. Sodium borohydride and isopropyl alcohol (IPA) were purchased from Samchun Chemical. 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl was purchased from Acros Organics. Cesium carbonate and Pt/C catalyst were purchased from Alfa Aesar. 6,6′-Dibromo-2,2′-bis(hexyloxy)-1,1′-binaphthalene were prepared according to published procedures.18 All other chemicals were reagent grade and used as received.Instrumentation
1H NMR spectra were recorded using a 300 or 400 MHz spectrometer. Chemical shifts are reported in ppm from tetramethylsilane with the solvent resonance as the internal standard (CDCl3: δ 7.27 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constants (Hz), and integration. 13C NMR spectra were recorded using a 75 or 100 MHz spectrometer with complete proton decoupling. Chemical shifts are reported in ppm from tetramethylsilane with the solvent resonance as the internal standard (CDCl3: δ 77.26 ppm). High-resolution mass spectra (HRMS) were obtained using an electrospray ionization (ESI) time-of-flight mass spectrometer. Low-resolution mass spectra (LRMS) were obtained using an electron ionization (EI) time-of-flight mass spectrometer. Melting points were determined using a melting point apparatus and are uncorrected. Unless otherwise noted, all the reactions were carried out in freshly distilled solvents under an atmosphere of dry N2 in oven-dried (130 °C) glassware. N,N′-Dimethylformamide was distilled over magnesium sulfate, and tetrahydrofuran was distilled over sodium benzophenone ketyl prior to use, unless otherwise specified. All work-up and purification procedures were carried out with reagent grade solvents in the air.Electrochemical characterization
The electrochemical characterization was investigated using a potentiostat. A standard three-electrode electrochemical cell equipped with a glassy carbon rotating disc electrode (RDE), a Pt electrode, a Ag/AgCl reference electrode, and a Pt-net counter electrode. A catalyst-coated glassy carbon electrode (GCE) or Pt electrode was used as the working electrode. Before each measurement, the electrode was polished with a 0.05 μm alumina paste followed by washing with distilled (DI) water. All the potentials reported are with respect to the Reversible Hydrogen Electrode (RHE).Preparation of catalyst inks
Catalyst inks were prepared by mixing commercial Pt/C catalyst (40 wt% Pt/C, 10 mg), Nafion® solution (5 wt%, 100 μL), and isopropyl alcohol (IPA, 1000 μL). The prepared ink (∼6 μL) was coated on the GCE with a diameter of 5 mm.Cyclic voltammetry (CV) measurements
For CV measurements, the working electrode was pretreated electrochemically before each measurement. The binaphthyl thiol-treated Pt electrodes were prepared by immersing the pristine Pt electrodes in 0.13 mM solutions of BNSHs for ∼1 min, and then the electrodes were rinsed with DI water. CVs were recorded in a N2-purged 0.1 M HClO4 solution with 0.01 M H3PO4 at a scan rate of 50 mV s−1.Measurements of ORR activity
The ORR activities were measured in 0.1 M HClO4 and 0.01 M H3PO4 solutions under O2 using the glassy carbon rotating disk electrode (RDE) at a rotation and sweep rates of 1600 rpm and 10 mV s−1, respectively. To produce a clean electrode surface, several potential sweeps between 0.6 and 1.1 V versus RHE were applied to the electrode prior to the ORR measurement. In the ORR polarization curve, current densities were normalized with reference to the geometric area of the glassy carbon RDE (0.196 cm2).For the ORR at a RDE, the Koutecky–Levich equation can be described as follows:
For each catalyst, the kinetic current was normalized to the real surface area (0.196 cm2).
Representative experimental procedure for the synthesis of dithiol compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain product 2a (209 mg, 0.347 mmol, 59%) as a pale yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.95 (s, 2H), 7.91 (d, J = 9.0 Hz, 2H), 7.41 (d, J = 9.0 Hz, 2H), 7.15 (s, 4H), 3.93 (t, J = 6.6 Hz, 4H), 2.42 (s, 6H), 1.40 (t, J = 6.3 Hz, 4H), 1.08–0.89 (m, 12H), 0.74 (t, J = 6.9 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 194.7, 155.5, 134.4, 134.1, 131.0, 129.4, 129.2, 126.3, 122.24, 119.7, 115.7, 69.4, 31.3, 30.1, 29.2, 25.3, 22.4, 13.9; LRMS (EI) m/z: [M+] calcd for C36H42O4S2 602, found 602.
1) to obtain product 2a (209 mg, 0.347 mmol, 59%) as a pale yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.95 (s, 2H), 7.91 (d, J = 9.0 Hz, 2H), 7.41 (d, J = 9.0 Hz, 2H), 7.15 (s, 4H), 3.93 (t, J = 6.6 Hz, 4H), 2.42 (s, 6H), 1.40 (t, J = 6.3 Hz, 4H), 1.08–0.89 (m, 12H), 0.74 (t, J = 6.9 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 194.7, 155.5, 134.4, 134.1, 131.0, 129.4, 129.2, 126.3, 122.24, 119.7, 115.7, 69.4, 31.3, 30.1, 29.2, 25.3, 22.4, 13.9; LRMS (EI) m/z: [M+] calcd for C36H42O4S2 602, found 602.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain thiol product BNSH (340 mg, 0.655 mmol, 92%) as a pale yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.78 (s, 2H), 7.77 (d, J = 9.0 Hz, 2H), 7.36 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 3.96–3.87 (m, 4H), 3.48 (s, 2H), 1.38 (t, J = 7.0 Hz, 4H), 1.09–0.90 (m, 12H), 0.75 (t, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 154.3, 132.2, 129.5, 128.6, 128.0, 127.8, 126.2, 124.6, 120.2, 116.3, 69.6, 31.3, 29.3, 25.3, 22.4, 13.9; LRMS (EI) m/z: [M+] calcd for C32H38O2S2 518, found 518.
1) to obtain thiol product BNSH (340 mg, 0.655 mmol, 92%) as a pale yellow oil. 1H NMR (300 MHz, CDCl3): δ 7.78 (s, 2H), 7.77 (d, J = 9.0 Hz, 2H), 7.36 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 3.96–3.87 (m, 4H), 3.48 (s, 2H), 1.38 (t, J = 7.0 Hz, 4H), 1.09–0.90 (m, 12H), 0.75 (t, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 154.3, 132.2, 129.5, 128.6, 128.0, 127.8, 126.2, 124.6, 120.2, 116.3, 69.6, 31.3, 29.3, 25.3, 22.4, 13.9; LRMS (EI) m/z: [M+] calcd for C32H38O2S2 518, found 518.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O/NH3 solution and extracted with ethyl acetate (3 × 10 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated, and purified by silica gel column chromatography (hexanes/EtOAc, 8
1 H2O/NH3 solution and extracted with ethyl acetate (3 × 10 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated, and purified by silica gel column chromatography (hexanes/EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the product BNCN (19.0 mg, 0.0377 mmol, 23%) as a white powder. 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 2H), 8.02 (d, J = 9.0 Hz, 2H), 7.52 (d, J = 9.0 Hz, 2H), 7.34 (d, J = 8.8 Hz, 2H), 7.15 (d, J = 8.8 Hz, 2H), 4.05–3.95 (m, 4H), 1.50–1.35 (m, 4H), 1.10–0.85 (m, 12H), 0.75 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 157.0, 135.5, 134.4, 130.4, 127.7, 126.8, 126.2, 119.6, 119.1, 116.1, 106.7, 69.1, 31.1, 28.9, 25.2, 22.3, 13.7; LRMS (EI) m/z: [M]+ calcd for C34H36N2O2 504, found 504.
1) to obtain the product BNCN (19.0 mg, 0.0377 mmol, 23%) as a white powder. 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 2H), 8.02 (d, J = 9.0 Hz, 2H), 7.52 (d, J = 9.0 Hz, 2H), 7.34 (d, J = 8.8 Hz, 2H), 7.15 (d, J = 8.8 Hz, 2H), 4.05–3.95 (m, 4H), 1.50–1.35 (m, 4H), 1.10–0.85 (m, 12H), 0.75 (t, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 157.0, 135.5, 134.4, 130.4, 127.7, 126.8, 126.2, 119.6, 119.1, 116.1, 106.7, 69.1, 31.1, 28.9, 25.2, 22.3, 13.7; LRMS (EI) m/z: [M]+ calcd for C34H36N2O2 504, found 504.Results and discussion
To prevent the phosphate ion-poisoning on the Pt surface in phosphoric acid fuel cells (PAFCs) or high-temperature proton exchange membrane fuel cells (HT-PEMFCs), BNSHs with a tweezer-like structure and thiols were designed for self-assembly on Pt surface through thiol–Pt interactions (Scheme 1). In binaphthyl, two naphthalene moieties are connected through the C–C covalent bond, and those naphthalenes cannot be positioned flat, because of the hindered rotation around the C–C bond. Thus, the naphthalenes exist perpendicular to each other and form a tweezer-like structure, which was found to be advantageous for physically blocking phosphate ions from the Pt surface (Fig. 1b). Various barrier molecules with binaphthyls were synthesized to investigate the effect of molecular structure on the ORR activities. First, binaphthyl dithiol (BNSH) was prepared from commercially available 6,6′-dibromo-1,1′-bi-2-naphthol, which was alkylated with hexyl group (C6). Then, the bromo groups were converted to acetyl thioester through lithiation and sulfur addition, followed by acetylation in moderate overall yields. Direct isolation of thiol compounds after sulfur addition was unsuccessful. Instead, thioester compounds were isolated, and then the acetyl groups were deprotected to furnish the target BNSH. To investigate the effect of alkyl chains, ethyl and dodecyl derivatives, C2- and C12-BNSH, respectively, were synthesized via similar methods. | ||
| Scheme 1 Synthesis of BNSHs and derivatives as the barrier molecules for PA-poisoning on Pt surface. | ||
The number of thiols groups may affect the self-assembly of the molecules, and thus mono-thiol derivative, BN-1-SH, was synthesized. In this case, the synthesis started with 1,1′-bi-2-naphthol, as only one naphthalene is needed to functionalize. One of the two naphthol groups was protected with pivaloyl group, which was deactivated in the bromination reaction. After the bromination of the other naphthol moiety, the pivaloyl group was de-protected, and then both naphthols were alkylated with hexyl groups. Using the similar thiolation method as described, BN-1-SH was prepared in moderate yield. Cyanide-functionalized version, BNCN, was synthesized via aromatic cyanation with CuCN to investigate the role of thiol groups. Lastly, the importance of the tweezer-like structure was demonstrated by comparison of the results with the naphthol version, NSH, prepared by similar methods: alkylation of 6-bromo-2-naphthol and subsequent thiolation.23,24 All BNSHs and derivatives that were newly synthesized were characterized by 1H and 13C-NMR spectroscopy and mass spectroscopy, which confirm their molecular structures. Especially, the presence of thiol groups was evident in 1H-NMR spectra; the chemical shifts of thiols were clearly observed at 3.48–3.53 ppm from tetramethylsilane.
The barrier BNSHs were tested by measuring the ORR activities of a Pt catalyst in the presence of PA. Pt nanoparticles (∼2.3 nm) on carbon as the catalyst were utilized, following the literature procedures.25 Briefly, the catalyst ink was prepared by mixing commercial 40 wt% Pt/C catalyst and 5 wt% Nafion solution in isopropyl alcohol. The prepared ink was drop-coated on a glassy carbon electrode (GCE) and dried. Then, the electrode was dipped in a THF solution of molecular barrier materials (0.13 mM) for 1 min. For the measurements, aqueous solutions of 0.1 M HClO4 were used as the electrolytes.
When the ORR polarization curves in the presence (0.01 M) and absence of PA (Fig. 1d) was compared, the kinetic current density (jk@0.8 V) decreased to 11 mA cm−2 with PA, while that of the pristine Pt was 22 mA cm−2 (Fig. 1). The kinetic current density was measured at 0.8 V versus RHE. This result strongly suggests that phosphate ions bind to the surface of Pt nanoparticles, thereby decreasing the catalytic activities. The binding of phosphate ions also reduced the hydrogen underpotential deposition (UPD) at <0.3 V (versus RHE) in the cyclic voltammogram (CV, Fig. 1d), which was companied by reduced OH adsorption at >0.6 V (versus RHE).
Interestingly, when the Pt catalyst was treated with BNSH (0.13 mM, 1 min), the ORR activity significantly increased (Fig. 1d and e) in the presence of PA. Because BNSHs can be adsorbed on to the surface of Pt via Pt–S interactions, the long-time exposure of Pt to the BNSH solution resulted in its excessive coverage over the Pt surface, thus substantially decreased the ORR activity of the Pt catalyst (Fig. S2†). However, when the BNSH treatment was limited to only 1 min, a significant enhancement in the ORR activity was observed (jk@0.8 V = 20 mA cm−2). This enhancement was quite interesting, as it was measured in the presence of poisonous PA (0.01 M). We speculate that the BNSH molecules were adsorbed sparsely on the surface of Pt nanoparticles such that the approach of phosphate anions was physically blocked, while the smaller oxygen molecules can approach rather freely. It is noteworthy that the simple treatment (dipping) of BNSHs can effectively enhance the ORR activity of Pt in the presence of phosphate anions.
The structural features of the BNSH molecule attributing to the enhanced ORR activity in the presence of PA were investigated. BNSH molecule has three distinctive features: (1) the tweezer-like binaphthyl, (2) thiol functional groups for binding on Pt, and (3) alkoxy side chains. First, the effect of the tweezer-like structure of binaphthyls was compared to the result from mono-naphthyl derivative NSH. NSH is almost half the BNSH and contains the same thiol and hexyloxy side chains, but the main aromatic group is a naphthalene instead of binaphthyl. Thus, it cannot create a void around the molecule. Pt nanoparticles were treated with NSH (0.13 mM, 1 min) under the similar conditions of BNSH and subjected to the ORR measurements in the presence of PA (0.01 M) (Fig. 1). Contrary to the case of BNSH, the enhancement of ORR activity was not observed, and the kinetic current density (jk@0.8 V) further decreased to 7 mA cm−2, which is worse than that of the pristine Pt in the presence of PA. At the same time, a considerable decrease in hydrogen UPD was observed in CV (Fig. 1d). We suspect that the same treatment condition resulted in more adsorption of NSH molecules on Pt surface, thereby decreasing the ORR activity. The flat aromatic ring of naphthalene may induce molecules' assembly through π–π interaction. This result emphasizes the importance of the tweezer-like structure of binaphthyls for the enhancement of ORR activities, although it cannot directly prove the hypothesis that oxygen molecules can go through the voids created by the binaphthyls.
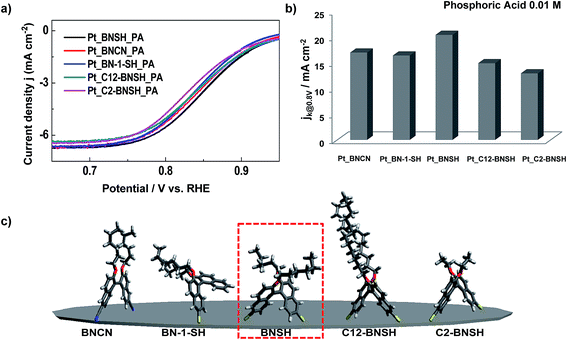
Next, the effect of thiol functional groups was investigated by comparing the results of the mono-thiol derivative BN-1-SH and cyanide derivative BNCN. The structure and conformation such as alkyloxy chains and the tweezer form of BNCN are similar to that of BNSH, but the binding group is different. We chose the cyanide group because cyanide ions are known to form an ensemble on Pt surface.17 When subjected to the similar conditions as BNSH, the BNCN-treated Pt catalyst showed the enhanced ORR activity (jk@0.8 V = 17 mA cm−2) in the presence of PA, but not as great as that of BNSH (Fig. 2a and b). This result is interesting as it clearly shows that the adsorption of binaphthyl molecules enhanced the ORR activity. The decrease in the ORR activity than that of BNSH is attributed to different binding affinities of cyanide groups (Fig. 2), although the detailed mechanism requires further investigation. In the case of the BN-1-SH with only one thiol group for binding on the Pt surface, the observed ORR activity was similar to the case of BNCN. We suspect that the binding affinity of BN-1-SH decreased because of one thiol group instead of two, thus decreasing the blocking of phosphate ions' approach. The analysis of the exact conformation of BN-1-SH on the Pt surface needs further investigation.
Lastly, the effect of the alkyl chain length was investigated by testing binaphthyl molecules C12-BNSH and C2-BNSH, with a tweezer-like structure but different alkyl chains (Scheme 1 and Fig. 2). Interestingly, the kinetic current density (jk@0.8 V) of the ORR polarization curves appeared to depend on the alkyl chain length. When the similar conditions were applied, the ORR activities of C12-BNSH and C2-BNSH showed somewhat enhanced compared to the case of the pristine Pt in the presence of PA. However, the enhancements were not as significant as that of BNSH. Although it is not easy to figure out the conformations of alkyl chains in the measurement conditions, the alkyl chains were expected to help in preventing the approach of phosphate anions. If the alkyl chain is too short (C2), the blocking is not as efficient. If it is too long (C12), however, the hydrophobic chains may aggregate, preventing the approach of oxygen molecules. The medium size of alkyl chain (C6) is appropriate for the blockage of the approach of phosphate anion only.
Conclusions
Binaphthyl thiols (BNSHs) were designed and synthesized as molecular barrier materials for preventing poisoning by phosphate anions in HT-PEMFCs. The reduced ORR activity of Pt nanoparticles in the presence of PA was restored by simply treating with BNSHs, and such enhancements depended on the molecular features of BNSHs such as a tweezer form, binding groups, and the length of alkyl chains. Without the tweezer structure, NSH showed the worst ORR activity in the presence of PA. Reduced binding affinity was observed by changing the functional group to cyanide (BNCN) or with only one thiol group (BN-1-SH), slightly enhancing the ORR activities. The investigation of the alkyl chain length showed that the medium-sized length (C6) was more efficient than short (C2) or long (C12) alkyl chains. The treatment of BNSHs by simple dipping provides an effective way of preventing PA poisoning on Pt catalysts for highly efficient HT-PEMFC.Acknowledgements
This study was supported by Civil-Military Technology Cooperation Program of Korea funded by the Ministry of Trade, Industry & Energy. This work was also partially supported by Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A02062095) and funded by the Ministry of Science, ICT and Future Planning (MSIP) (NRF-2010-0027963).References
- F. A. de Bruijn, V. A. T. Dam and G. J. M. Janssen, Fuel Cells, 2008, 8, 3–22 CrossRef CAS.
- A. Chandan, M. Hattenberger, A. El-kharouf, S. Du, A. Dhir, V. Self, B. G. Pollet, A. Ingram and W. Bujalski, J. Power Sources, 2013, 231, 264–278 CrossRef CAS.
- L. Dubau, L. Castanheira, F. Maillard, M. Chatenet, O. Lottin, G. Maranzana, J. Dillet, A. Lamibrac, J.-C. Perrin, E. Moukheiber, A. ElKaddouri, G. De Moor, C. Bas, L. Flandin and N. Caqué, Wiley Interdiscip. Rev.: Energy Environ., 2014, 3, 540–560 CAS.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- E. Antolini, Appl. Catal., B, 2009, 88, 1–24 CrossRef CAS.
- G. Álvarez, F. Alcaide, P. L. Cabot, M. J. Lázaro, E. Pastor and J. Solla-Gullón, Int. J. Hydrogen Energy, 2012, 37, 393–404 CrossRef.
- S. Subianto, Polym. Int., 2014, 63, 1134–1144 CrossRef CAS.
- M. Rikukawa and K. Sanui, Prog. Polym. Sci., 2000, 25, 1463–1502 CrossRef CAS.
- Q. Li, R. He, J. O. Jensen and N. J. Bjerrum, Fuel Cells, 2004, 4, 147–159 CrossRef CAS.
- Y. Zhai, H. Zhang, D. Xing and Z.-G. Shao, J. Power Sources, 2007, 164, 126–133 CrossRef CAS.
- R. Zeis, Beilstein J. Nanotechnol., 2015, 6, 68–83 CrossRef PubMed.
- Q. He, X. Yang, W. Chen, S. Mukerjee, B. Koel and S. Chen, Phys. Chem. Chem. Phys., 2010, 12, 12544–12555 RSC.
- E. Heider, N. Ignatiev, L. Jörissen, A. Wenda and R. Zeis, Electrochem. Commun., 2014, 48, 24–27 CrossRef CAS.
- K. L. Hsueh, E. R. Gonzalez, S. Srinivasan and D. T. Chin, J. Electrochem. Soc., 1984, 131, 823–828 CrossRef CAS.
- F. Zhou, S. J. Andreasen, S. K. Kær and D. Yu, Int. J. Hydrogen Energy, 2015, 40, 2833–2839 CrossRef CAS.
- S. Kaserer, K. M. Caldwell, D. E. Ramaker and C. Roth, J. Phys. Chem. C, 2013, 117, 6210–6217 CrossRef CAS.
- D. Strmcnik, M. Escudero-Escribano, K. Kodama, R. StamenkovicVojislav, A. Cuesta and N. M. Marković, Nat. Chem., 2010, 2, 880–885 CrossRef CAS PubMed.
- W. E. Kowtoniuk, M. E. Rueffer and D. K. MacFarland, Tetrahedron: Asymmetry, 2004, 15, 151–154 CrossRef CAS.
- S. J. Lee and W. Lin, J. Am. Chem. Soc., 2002, 124, 4554–4555 CrossRef CAS PubMed.
- K. Kawabata and H. Goto, J. Mater. Chem., 2012, 22, 23514–23524 RSC.
- Y. Li and Q. Li, Org. Lett., 2012, 14, 4362–4365 CrossRef CAS PubMed.
- J. C. Ostrowski, J. R. A. Hudack, M. R. Robinson, S. Wang and G. C. Bazan, Chem.–Eur. J., 2001, 7, 4500–4511 CrossRef CAS.
- C. Gao, S. Silvi, X. Ma, H. Tian, A. Credi and M. Venturi, Chem.–Eur. J., 2012, 18, 16911–16921 CrossRef CAS PubMed.
- D.-C. Jeong, H. Lee, K. S. Yang and C. Song, Macromolecules, 2012, 45, 9571–9578 CrossRef CAS.
- Z.-Y. Zhou, X. Kang, Y. Song and S. Chen, J. Phys. Chem. C, 2012, 116, 10592–10598 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13123a |
| ‡ These two authors contributed equally to this work. |
| § Present address: Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, USA. |
| This journal is © The Royal Society of Chemistry 2016 |