A novel adduct of ECG fused to piceid and four new dimeric stilbene glycosides from Polygonum cuspidatum†
Ya-nan Yang‡
,
Fu-shuang Li‡,
Fu Liu,
Zi-ming Feng,
Jian-shuang Jiang and
Pei-cheng Zhang*
State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, People's Republic of China. E-mail: pczhang@imm.ac.cn
First published on 17th June 2016
Abstract
Polyflavanostilbene B (1), an unusual adduct of epicatechin-3-O-gallate fused to piceid through a carbon–carbon bond, four new dimeric stilbene glycosides (2–5), three new stilbene glucosides (6–8), one new flavan glucoside (9), and six known compounds were isolated from the rhizome of Polygonum cuspidatum. The structures of these compounds were elucidated using spectroscopic data, including electronic circular dichroism (ECD) and Rh2(OCOCF3)4-induced CD spectra. All of the compounds were screened for their inhibitory activity against α-glucosidase using acarbose as a positive control (IC50 = 385 μM), and strong inhibitory activity against α-glucosidase was observed for compound 8 (IC50 = 3.04 μM).
Introduction
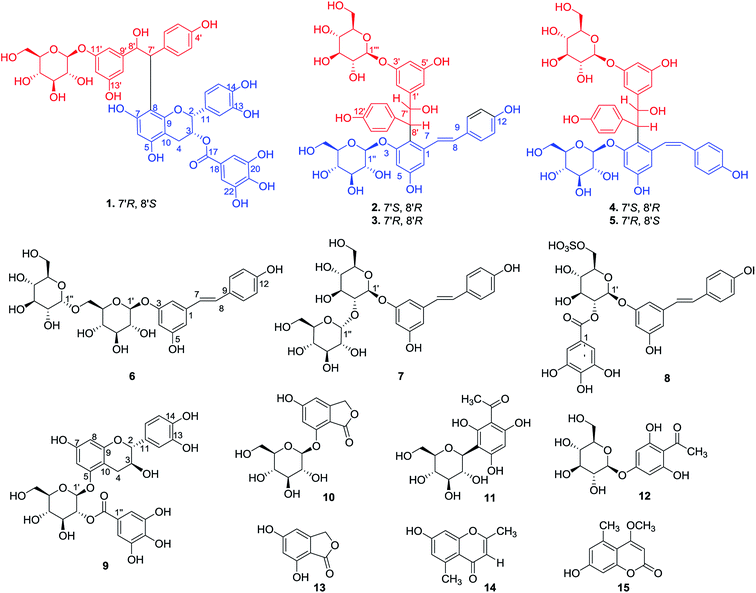
The genus Polygonum comprises more than 300 species around the world and approximately 120 species distributed in China. The rhizome of Polygonum cuspidatum, named HuZhang in China, is a traditional Chinese medicine (TCM) that has been documented in the Chinese Pharmacopoeia (1977–2015 editions), and it is conventionally used for the treatment of diabetes, inflammation, hyperlipemia, and infection.1,2 Modern pharmacological studies on P. cuspidatum have revealed significant biological activity, including antioxidant,3 antiviral,4 antibacterial,5 antitumour,6 and neuroprotective effects.7 Various anthroquinones,8 stilbenes,9 flavonoids,10 and phenols,11 have been isolated from this plant. In particular, stilbenes (resveratrol and piceid) and anthraquinones are considered to be the major constituents of P. cuspidatum and are generally regarded as the index for quality assessment of this herb.12Previously, we investigated the bioactive components of the rhizome of P. cuspidatum. This study revealed a novel adduct of epicatechin-3-O-gallate and piceid known as polyflavanostilbene A,13 two novel naphthalene-fused piceid glycosides, and a series of naphthalene glucosides.14 In the current study, we report the isolation of an unusual adduct of epicatechin-3-O-gallate fused to piceid through a carbon–carbon bond (1), four new dimeric stilbene glycosides (2–5), three new stilbene glucosides (6–8), and one new flavan glucoside (9), as well as six known compounds (10–15) (Fig. 1). The structures of these compounds were using spectroscopic data (1D and 2D NMR, UV, IR, ORD, HRESIMS, ECD, and Rh2(OCOCF3)4-induced CD) and comparison to literature data. Additionally, the inhibitory activities of compounds 1–15 against α-glucosidase were examined. Compound 8 exhibited strong inhibitory activity against α-glucosidase with an IC50 value of 3.04 μM.
Results and discussion
Compound 1 was isolated as a white powder. Its molecular formula was determined as C42H40O19 from the HRESIMS ion at m/z 847.2094 [M − H]− (calcd for C42H39O19: 847.2091), indicating 23 degrees of unsaturation. IR absorptions revealed the presence of hydroxyl (3372 cm−1) and carbonyl (1613 cm−1) groups, in addition to aromatic rings (1513 and 1450 cm−1).The 1H NMR spectrum (Table 1) of 1 displayed a set of AA′BB′ system aromatic protons at δH 7.04 (2H, d, J = 8.5 Hz) and 6.53 (2H, d, J = 8.5 Hz), three meta-coupled aromatic protons at δH 6.18 (1H, s), 6.15 (1H, s), and 6.12 (1H, s), three ABX system aromatic protons at δH 6.96 (1H, dd, J = 8.5, 1.5 Hz), 6.90 (1H, d, J = 1.5 Hz), and 6.68 (1H, d, J = 8.5 Hz), a singlet aromatic proton at δH 6.00 (1H, s), and two characteristic signals of a galloyl moiety at δH 6.88 (2H, s). In addition, four methine protons at δH 4.45 (1H, d, J = 10.5 Hz), 5.39 (1H, dd, J = 10.5, 4.0 Hz), 5.18 (1H, br s), and 5.36 (1H, br s) and two methylene protons at δH 2.76 (1H, dd, J = 17.5, 4.5 Hz) and 3.03 (1H, d, J = 17.5 Hz) were observed. Finally, a doublet at δH 4.61 (1H, d, J = 7.5 Hz) caused by an anomeric proton and the proton signals between δH 3.15 and 3.64 indicated the presence of a glycosyl group. After cellulose hydrolysis, the sugar unit of 1 was confirmed to be the D-configuration by GC analysis of its trimethylsilyl L-cysteine derivative.
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 5.18, br s | ||||
| 3 | 5.36, br s | ||||
| 4 | 3.03, d (17.5) | 6.21, s | 6.13, s | 6.44, s | 6.48, s |
| 2.76, dd (17.5, 4.5) | |||||
| 6 | 6.00, s | 6.54, s | 6.27, s | 6.06, s | 6.06, s |
| 7 | 6.70, d (16.0) | 6.59, d (16.0) | 6.60, d (12.0) | 6.58, d (11.5) | |
| 8 | 7.27, d (16.0) | 6.87, d (16.0) | 6.51, d (12.0) | 6.51, d (11.5) | |
| 9 | |||||
| 10 | 7.34, d (8.0) | 7.35, d (8.5) | 6.86, d (8.5) | 6.87, d (8.5) | |
| 11 | 6.79, d (8.0) | 7.01, d (8.5) | 6.47, d (8.5) | 6.46, d (8.5) | |
| 12 | 6.90, d (1.5) | ||||
| 13 | 6.79, d (8.0) | 7.01, d (8.5) | 6.47, d (8.5) | 6.46, d (8.5) | |
| 14 | 7.34, d (8.0) | 7.35, d (8.5) | 6.86, d (8.5) | 6.87, d (8.5) | |
| 15 | 6.68, d (8.5) | ||||
| 16 | 6.96, dd (8.5, 1.5) | ||||
| 19 | 6.88, s | ||||
| 23 | 6.88, s | ||||
| 1′ | |||||
| 2′ | 7.04, d (8.5) | 6.40, s | 6.49, s | 6.41, s | 6.23, s |
| 3′ | 6.53, d (8.5) | ||||
| 4′ | 6.23, s | 6.25, s | 6.20, s | 6.16, s | |
| 5′ | 6.53, d (8.5) | ||||
| 6′ | 7.04, d (8.5) | 6.53, s | 6.40, s | 6.27, s | 6.32, s |
| 7′ | 4.45, d (10.5) | 5.57, d (10.0) | 5.49, d (3.0) | 5.61, d (10.5) | 5.66, d (10.5) |
| 8′ | 5.39, dd (10.5, 4.0) | 4.37, d (10.0) | 4.47, m | 4.49, d (10.5) | 4.29, d (10.5) |
| 10′ | 6.15, s | 6.89, d (8.0) | 7.03, d (8.5) | 6.94, d (8.0) | 7.10, d (8.0) |
| 11′ | 6.43, d (8.0) | 6.54, d (8.5) | 6.34, d (8.0) | 6.33, d (8.0) | |
| 12′ | 6.18, s | ||||
| 13′ | 6.43, d (8.0) | 6.54, d (8.5) | 6.34, d (8.0) | 6.33, d (8.0) | |
| 14′ | 6.12, s | 6.89, d (8.0) | 7.03, d (8.5) | 6.94, d (8.0) | 7.10, d (8.0) |
| 1′′ | 4.61, d (7.5) | 4.85, d (7.0) | 4.87, d (7.5) | 4.86, d (7.5) | 5.02, d (7.0) |
| 2′′ | 3.15, m | 3.46, m | 3.19, m | 3.46, m | 3.50, m |
| 3′′ | 3.21, m | 3.36, m | 3.32, m | 3.34, m | 3.33, m |
| 4′′ | 3.25, m | 3.27, m | 3.17, m | 3.27, m | 3.23, m |
| 5′′ | 3.21, m | 3.21, m | 3.42, m | 3.19, m | 3.34, m |
| 6′′ | 3.55, m | 3.52, m | 3.46, m | 3.54, m | 3.51, m |
| 3.64, m | 3.75, d (11.0) | 3.66, m | 3.73, m | 3.73, m | |
| 1′′′ | 4.66, d (7.0) | 4.57, d (6.0) | 4.63, d (7.5) | 4.57, d (7.5) | |
| 2′′′ | 3.16, m | 3.15, m | 3.14, m | 3.13, m | |
| 3′′′ | 3.36, m | 3.33, m | 3.20, m | 3.19, m | |
| 4′′′ | 3.20, m | 3.31, m | 3.18, m | 3.19, m | |
| 5′′′ | 3.22, m | 3.19, m | 3.43, m | 3.19, m | |
| 6′′′ | 3.54, m | 3.46, m | 3.50, m | 3.51, m | |
| 3.65, d (11.5) | 3.66, m | 3.61, d (11.5) | 3.64, m |
The 13C NMR spectrum (Table 2) of 1 revealed a total of 42 carbon signals, six of which were assigned to an O-glucose unit; the remaining 36 carbons were assigned to a C6–C2–C6 unit and a flavan aglycone with an additional galloyl moiety. A careful comparison of the 1D NMR signals of 1 with the corresponding data of epicatechin-3-O-gallate (ECG),15 suggested the presence of an ECG moiety in 1, which was verified by HMBC correlations from H-3 to C-2, C-4, C-10, C-11, and C-17, and from H-19, H-23 to C-17. By considering the remaining eight degrees of unsaturation and the molecular formula, the C6–C2–C6 unit was elucidated as the 4′,8′,11′,13′-tetrahydroxyl diphenylethane moiety. The connectivity of these two moieties was established by analysing the HMBC spectrum (Fig. 2). Correlations from H-7′ to C-7, C-8, and C-9 were observed, suggesting that the C-7′ is connected to the C-8 of the ECG moiety by a C–C bond. The sugar moiety was confirmed to be at C-11′ position of the aglycone by the HMBC correlation of H-1′′ with C-11′.
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 139.7 | 140.0 | 141.0 | 140.6 | 139.4 | 139.4 | 140.1 | ||
| 2 | 76.8 | 120.4 | 117.1 | 121.7 | 122.1 | 105.6 | 105.2 | 107.9 | 81.4 |
| 3 | 68.8 | 157.1 | 157.7 | 157.7 | 156.3 | 158.9 | 158.5 | 159.0 | 66.0 |
| 4 | 26.3 | 101.8 | 104.0 | 102.2 | 102.6 | 102.9 | 103.2 | 103.5 | 28.0 |
| 5 | 153.7 | 156.1 | 157.0 | 156.7 | 155.9 | 158.4 | 158.4 | 158.8 | 155.7 |
| 6 | 95.6 | 106.9 | 105.0 | 109.6 | 109.5 | 106.6 | 107.5 | 105.8 | 94.9 |
| 7 | 154.2 | 124.7 | 126.4 | 129.0 | 128.3 | 125.3 | 125.2 | 125.6 | 156.8 |
| 8 | 108.6 | 130.7 | 130.0 | 130.2 | 129.6 | 128.5 | 128.6 | 128.5 | 96.6 |
| 9 | 154.4 | 128.2 | 131.0 | 127.9 | 127.3 | 128.1 | 128.0 | 129.1 | 155.2 |
| 10 | 97.9 | 127.8 | 127.6 | 130.9 | 130.6 | 128.1 | 128.0 | 128.6 | 101.2 |
| 11 | 130.2 | 115.8 | 116.6 | 115.3 | 114.7 | 115.6 | 115.6 | 115.9 | 130.2 |
| 12 | 114.3 | 157.4 | 157.0 | 157.1 | 157.5 | 157.4 | 157.4 | 157.7 | 115.2 |
| 13 | 144.5 | 115.8 | 116.6 | 115.3 | 114.7 | 115.6 | 115.6 | 115.9 | 145.0 |
| 14 | 144.9 | 127.8 | 127.6 | 130.9 | 130.6 | 128.1 | 128.0 | 128.6 | 144.9 |
| 15 | 115.2 | 114.9 | |||||||
| 16 | 117.5 | 118.7 | |||||||
| 17 | 165.5 | ||||||||
| 18 | 119.8 | ||||||||
| 19 | 108.9 | ||||||||
| 20 | 145.6 | ||||||||
| 21 | 138.7 | ||||||||
| 22 | 145.6 | ||||||||
| 23 | 108.9 | ||||||||
| 1′ | 133.7 | 147.8 | 146.7 | 148.5 | 148.0 | 100.8 | 100.6 | 99.4 | 97.9 |
| 2′ | 129.6 | 106.8 | 105.9 | 107.2 | 106.8 | 73.2 | 75.0 | 74.0 | 73.5 |
| 3′ | 114.5 | 158.1 | 158.4 | 158.6 | 157.7 | 76.7 | 77.4 | 74.4 | 74.2 |
| 4′ | 154.8 | 102.0 | 102.0 | 102.1 | 101.5 | 69.7 | 69.9 | 70.8 | 69.7 |
| 5′ | 114.5 | 157.4 | 157.7 | 158.0 | 157.5 | 75.0 | 77.0 | 75.4 | 77.0 |
| 6′ | 129.6 | 108.6 | 107.4 | 109.5 | 107.9 | 66.3 | 60.5 | 66.1 | 60.4 |
| 7′ | 49.6 | 74.0 | 74.5 | 74.4 | 74.0 | ||||
| 8′ | 74.7 | 52.7 | 50.5 | 54.0 | 54.4 | ||||
| 9′ | 148.4 | 132.9 | 132.6 | 133.1 | 132.5 | ||||
| 10′ | 106.6 | 129.9 | 129.2 | 131.1 | 130.6 | ||||
| 11′ | 157.7 | 114.5 | 114.6 | 114.7 | 114.3 | ||||
| 12′ | 101.3 | 154.9 | 155.2 | 155.4 | 154.8 | ||||
| 13′ | 157.3 | 114.5 | 114.6 | 114.7 | 114.3 | ||||
| 14′ | 108.1 | 129.9 | 129.2 | 131.1 | 130.6 | ||||
| 1′′ | 100.5 | 101.5 | 100.4 | 101.2 | 100.6 | 98.5 | 97.6 | 119.8 | 119.6 |
| 2′′ | 73.3 | 74.4 | 73.3 | 74.0 | 73.3 | 72.1 | 72.1 | 109.2 | 108.9 |
| 3′′ | 76.7 | 77.0 | 77.1 | 77.0 | 77.1 | 73.2 | 73.2 | 146.0 | 145.5 |
| 4′′ | 69.2 | 69.6 | 69.8 | 69.6 | 69.8 | 70.2 | 70.1 | 139.0 | 138.5 |
| 5′′ | 76.7 | 76.6 | 76.9 | 76.6 | 77.0 | 72.6 | 72.2 | 146.0 | 145.5 |
| 6′′ | 60.3 | 60.6 | 60.5 | 60.3 | 60.8 | 60.8 | 60.7 | 109.2 | 108.9 |
| 7′′ | 165.4 | 165.0 | |||||||
| 1′′′ | 100.6 | 101.1 | 100.4 | 100.3 | |||||
| 2′′′ | 73.2 | 73.3 | 73.1 | 73.1 | |||||
| 3′′′ | 77.2 | 76.7 | 76.8 | 76.6 | |||||
| 4′′′ | 69.4 | 69.5 | 69.3 | 69.3 | |||||
| 5′′′ | 76.9 | 76.7 | 77.1 | 76.8 | |||||
| 6′′′ | 60.5 | 60.8 | 60.6 | 60.3 |
Examination of the ECD and Rh2(OCOCF3)4-induced CD spectra of 1 allowed for determination of the absolute configurations of C-2, C-3, C-7′, and C-8′. Cellulase hydrolysis of 1 resulted in 1a (the aglycone) and D-glucose. The 8′S configuration was supported by a positive Cotton effect at 357 nm in the Rh2(OCOCF3)4-induced CD spectrum of 1a (Fig. S7, ESI†).16 The threo configuration between the two chiral centres at C-7′ and C-8′ was established using the coupling constant (10.5 Hz) between H-7′ and H-8′.17 From the above analysis, the absolute configuration of C-7′ was determined to be R. In addition, the 2R,3R configurations were confirmed by the small coupling constant of H-2/H-3 and the negative Cotton effect at 280 nm in the ECD spectrum.18 Based on these results, the structure of 1 was assigned as shown in Fig. 1, and the compound was accorded the trivial name polyflavanostilbene B.
Compound 2 was obtained as a white amorphous powder, with a molecular formula of C40H44O17 on the basis of HRESIMS (m/z 819.2478 [M + Na]+, calcd for C40H44O17Na: 819.2471). Its 1H NMR spectrum exhibited two sets of AA′BB′ system aromatic protons at δH 7.34 (2H, d, J = 8.0 Hz), 6.89 (2H, d, J = 8.0 Hz), 6.79 (2H, d, J = 8.0 Hz), and 6.43 (2H, d, J = 8.0 Hz), three meta-coupled aromatic protons at δH 6.53 (1H, s), 6.40 (1H, s), and 6.23 (1H, s), one 1,2,3,5-tetrasubstituted aromatic protons at δH 6.54 (1H, s) and 6.21 (1H, s), two trans-olefinic protons at δH 7.27 (1H, d, J = 16.0 Hz) and 6.70 (1H, d, J = 16.0 Hz), two aliphatic protons at δH 5.57 (1H, d, J = 10.0 Hz) and 4.37 (1H, d, J = 10.0 Hz), and two glucopyranosyl anomeric protons at 4.85 (1H, d, J = 7.0 Hz) and 4.66 (1H, d, J = 7.0 Hz). The 13C NMR spectrum of 2 showed 40 carbon resonances (Table 2), consisting of 12 carbon signals of two glucose units and 28 skeleton carbon signals corresponding to two C6–C2–C6 units. In the HMBC spectrum (Fig. 2), the correlation peaks of H-8′ at δH 4.37 with C-1 (δC 139.7), C-2 (δC 120.4), and C-3 (δC 157.1) helped verify the linkage point between C-8′ and C-2. Furthermore, two glucose units were determined to be located at C-3 and C-3′ based on correlations between H-1′′ (δH 4.85) with C-3 (δC 157.1) and H-1′′′ (δH 4.66) with C-3′ (δC 158.1) in the HMBC spectrum. This spectroscopic data suggested that 2 had the same planar structure as dimeric stilbene glycoside 1,17 which was previously isolated from P. cuspidatum but the absolute configurations were not assigned. The absolute configurations of C-7′ and C-8′ were identified by the same method as that used for 1. The coupling constant (J7′,8′ = 10.0 Hz) confirmed the threo configuration of C-7′ and C-8′. Hydrolysis of 2 with cellulase yielded 2a and D-glucose, which was confirmed by GC analysis of its trimethylsilyl L-cysteine derivative. The absolute configuration of C-7′ in 2a was defined as S based on a positive Cotton effect at 350 nm in the Rh2(OCOCF3)4-induced CD spectrum (Fig. S15, ESI†). C-8′ was then assigned the R absolute configuration, in accordance with the 7′,8′-threo conformation. Thus, the structure of 2 was established as shown (Fig. 1), and this compound was given the trivial name stilbenedimer A.
The molecular formula of 3 was determined to be the same as 2 (C40H44O17), based on the positive HRESIMS ion observed at m/z 819.2464 [M + Na]+. Careful analysis of the UV, IR, and NMR spectroscopic data revealed that 3 had the same planar structure as 2. The coupling constant (3.0 Hz) of the two aliphatic protons H-7′ (δH 5.49) and H-8′ (δH 4.47) suggested that they were in the erythro conformation. Cellulase hydrolysis of 3 resulted in 3a and D-glucose. In the Rh2(OCOCF3)4-induced CD experiment (Fig. S23, ESI†), a negative Cotton effect at 361 nm indicated the 7′R,8′R configurations for 3a. Thus, 3 was established as shown and was named stilbenedimer B.
Compound 4 had the same molecular formula as 2, as indicated by the sodiated molecular ion peak observed at m/z 819.2463 [M + Na]+ in the HRESIMS. A characteristic coupling constant (J7,8 = 12.0 Hz) between H-7 and H-8 in the 1H NMR spectrum (Table 1) suggested the presence of a cis-stilbene moiety in 4 instead of the trans-stilbene moiety observed in 2. The presence of the aglycone (4a) was determined by the hydrolysis of 4 with cellulose. The threo configuration of C-7′ and C-8′ was verified by the coupling constant between H-7′ and H-8′ (J7′,8′ = 10.5 Hz). This result, combined with the positive Cotton effect at 358 nm in the Rh2(OCOCF3)4-induced CD spectrum of 4a (Fig. S31, ESI†), indicated the 7′S,8′R configuration of 4. Consequently, 4 was elucidated as an isomer of 2 and named stilbenedimer C.
Compound 5, a white amorphous powder, had the same molecular formula (C40H44O17Na, m/z 819.2468) as 4. The UV, IR, and NMR spectroscopy data of 5 indicated that it was an optical isomer of 4. Cellulase hydrolysis of 5 resulted in 5a and D-glucose. In the Rh2(OCOCF3)4-induced CD spectrum, a negative Cotton effect at 356 nm indicated the 7′R configuration in 5a (Fig. S39, ESI†). Meanwhile, the 8′S configuration was confirmed by the large coupling constant between H-7′ and H-8′ (J7′,8′ = 10.5 Hz). Based on the aforementioned information, 5 was identified as shown (Fig. 1) and named stilbenedimer D.
Compound 6 was obtained as a white amorphous powder and had a molecular formula of C26H32O13, as evidenced by the protonated molecular ion peak at m/z 553.1920 [M + H]+ (calcd for C26H33O13: 553.1916) in the HRESIMS. The UV, IR, and NMR spectroscopic data of 6 were closely related to those of piceid,18 a known compound that was isolated as the major component from the rhizome of P. cuspidatum. Comparison of the NMR spectra with those of piceid demonstrated these compounds differed only by the presence of an additional glucose moiety in 6. The 1H NMR spectrum displayed two anomeric protons at δH 4.78 (1H, d, J = 7.5 Hz) and δH 4.69 (1H, d, J = 3.0 Hz), which signified the presence of two glucose units with one β-linkage and one α-linkage. In the 13C NMR spectrum, the resonance for C-6′ (δC 66.3) of 6 was shifted significantly downfield compared to piceid. This suggested that the α-glucose was attached to C-6′ of the β-glucose, which was further supported by the HMBC correlation between H-1′′ and C-6′. After acid hydrolysis, the glucose unit was confirmed to be D-configuration by GC analysis of its trimethylsilyl L-cysteine derivative. Using the data above, the structure of 3 was elucidated and determined to be piceid-6′-O-α-D-glucopyranoside.
Compound 7 exhibited the same molecular formula (C26H32O13) as that of 6. Comparison of the NMR data for 7 and 6 showed that the two compounds differed by the location of the α-D-glucose unit. The key HMBC correlation from H-1′′ (δH 5.19) to C-2′ (δC 75.0) confirmed the location of the α-D-glucose unit at C-2′. All of the aforementioned data indicated that the structure of 7 was piceid-2′-O-α-D-glucopyranoside.
Compound 8 was obtained as a white amorphous powder. The molecular formula of 8 was C27H26O15S, as confirmed by the negative HRESIMS ion observed at m/z 621.0929 [M − H]− (calcd for C27H25O15S: 621.0920), and likely contains a sulfate group. A detailed analysis of the spectroscopic data revealed that 8 was a piceid derivative with a galloyl moiety. The HMBC correlation observed between H-2′ (δH 4.92) and C-7′′ (δC 165.4), together with the downfield shift of H-2′ (δH 4.92) indicated that the galloyl moiety was linked at C-2′ of glucose. The unusual downfield shift of the H-6′ (δH 4.16, 3.81), C-6′ (δC 66.1) was an important indicator that the sulfate group was attached at C-6′ of glucose. After acid hydrolysis, the sugar unit was confirmed to be D-glucose by GC analysis of its trimethylsilyl L-cysteine derivative. On the basis of these data, the structure of 8 was determined to be piceid-2′-galloyl-6′-sulfate.
Compound 9 was isolated as a white amorphous powder, and the molecular formula C28H28O15 was determined by the positive HRESIMS ion (m/z 627.1316 [M + Na]+). The IR spectrum revealed absorption bands attributed to hydroxy groups (3363 cm−1), carbonyl groups (1698 cm−1), and aromatic rings (1610, 1528, 1504, and 1453 cm−1). The 1H NMR spectrum (Table 3) of 9 revealed a set of ABX system aromatic protons at δH 6.67 (1H, d, J = 1.5 Hz), 6.63 (1H, d, J = 8.0 Hz) and 6.52 (1H, dd, J = 8.0, 1.5 Hz), two meta-coupled aromatic protons at δH 6.11 (1H, d, J = 1.5 Hz) and 5.85 (1H, d, J = 1.5 Hz), and two characteristic signals of a galloyl moiety at δH 6.94 (2H, s) in the downfield region. Additionally, two oxygenated methylene protons at δH 4.33 (1H, d, J = 7.5 Hz) and 3.59 (1H, m), two methylene protons at δH 2.45 (1H, overlap), 2.26 (1H, dd, J = 16.5, 8.5 Hz), and one anomeric proton at δH 5.11 (1H, d, J = 8.0 Hz) were also observed. The 13C NMR spectrum of 9 showed 28 carbon resonances (Table 2) corresponding to the glucose unit, galloyl moiety, and 3,5,7,3′,4′-pentahydroxyflavane. The HMBC correlation (Fig. 2) between H-2′ (δH 4.96) and C-7′′ (δC 165.0) indicated that the galloyl moiety was attached to C-2′ of the glucose. Furthermore, the linkage point of the glucose was identified by the correlation between the anomeric proton H-1′ and C-5 in the HMBC experiment. Acid hydrolysis of 9 gave the 3,5,7,3′,4′-pentahydroxyflavane (9a), gallic acid, and glucose. Using the same method as described in 8, the D-configuration of glucose was confirmed. The coupling constant of H-2 (J = 7.5 Hz) indicated that H-2/H-3 was in a trans configuration. This observation, along with the negative Cotton effect at 281 nm in the ECD spectrum of 9a (Fig. S64, ESI†), permits assignment of the absolute configurations of C-2 and C-3 as 2R,3S.19 Thus, 9 was defined as (+)-catechin-5-O-β-D-(2′-O-galloyl)-glucopyranoside.
| No. | 6 | 7 | 8 | 9 |
|---|---|---|---|---|
| 2 | 6.63, s | 6.75, s | 6.57, s | 4.33, d (7.5) |
| 3 | 3.59, m | |||
| 4 | 6.40, s | 6.33, s | 6.17, s | 2.26, dd (16.5, 8.5) |
| 2.45, overlap | ||||
| 6 | 6.59, s | 6.56, s | 6.59, s | 6.11, d (1.5) |
| 7 | 6.87, d (16.5) | 6.84, d (16.5) | 6.83, d (16.5) | |
| 8 | 6.99, d (16.5) | 7.02, d (16.5) | 6.93, d (16.5) | 5.85, d (1.5) |
| 10 | 7.41, d (8.5) | 7.39, d (8.5) | 7.42, d (8.5) | |
| 11 | 6.74, d (8.5) | 6.74, d (8.5) | 6.71, d (8.5) | |
| 12 | 6.67, d (1.5) | |||
| 13 | 6.74, d (8.5) | 6.74, d (8.5) | 6.71, d (8.5) | |
| 14 | 7.41, d (8.5) | 7.39, d (8.5) | 7.42, d (8.5) | |
| 15 | 6.63, d (8.0) | |||
| 16 | 6.52, dd (8.0, 1.5) | |||
| 1′ | 4.78, d (7.5) | 5.00, d (8.0) | 5.01, d (8.0) | 5.11, d (8.0) |
| 2′ | 3.44, m | 3.42, m | 4.92, dd (8.0, 8.0) | 4.96, dd (8.0, 8.0) |
| 3′ | 3.27, m | 3.50, m | 3.57, m | 3.58, m |
| 4′ | 3.27, m | 3.19, m | 3.26, m | 3.34, m |
| 5′ | 3.52, m | 3.35, m | 3.70, m | 3.43, m |
| 6′ | 3.62, m | 3.40, m | 3.81, m | 3.56, m |
| 3.75, m | 3.53, m | 4.16, d (10.0) | 3.72, d (11.0) | |
| 1′′ | 4.69, d (3.0) | 5.19, d (3.5) | ||
| 2′′ | 3.21, m | 3.90, m | 6.97, s | 6.94, s |
| 3′′ | 3.22, m | 3.44, m | ||
| 4′′ | 3.09, m | 3.35, m | ||
| 5′′ | 3.08, m | 3.19, m | ||
| 6′′ | 3.45, m | 3.53, m | 6.97, s | 6.94, s |
| 3.57, m | 3.72, m |
Based on MS data, NMR data and comparison with literatures, known compounds (Fig. 1) were identified as 7-O-(β-D-glucopyranosyloxy)-5-hydroxy-1(3H)-isobenzofuranone (10),20 2,4,6-trihydroxyacetophenone-3-C-β-D-glucopyranoside (11),21 2,4,6-trihydroxy-acetophenone-4-O-β-D-glucopyranoside (12),22 5,7-dihydroxyphthalide (13),20 altechromone A (14),23 and 7-demethylsiderin (15).24
Experimental
General experimental procedures
The optical rotations were measured on a Jasco P-2000 polarimeter (JASCO, Easton, MD, U.S.A.). ECD spectra were recorded with a JASCO J-815 spectrometer. IR spectra were recorded on a Nicolet 5700 spectrometer (Thermo Scientific, Waltham, MA, U.S.A.) using an FT-IR microscope transmission method. 1H NMR (500 MHz), 13C NMR (125 MHz), and 2D NMR spectra were run on Bruker 500 MHz NMR spectrometer (Bruker-Biospin, Billerica, MA, U.S.A.) using TMS as internal standard and the values are given in ppm. High-resolution electrospray ionization mass spectrometry (HRESIMS) was performed on an Agilent 1100 series LC/MSD ion trap mass spectrometer (Agilent Technologies, Waldbronn, Germany). HPLC-DAD analysis was performed using an Agilent 1200 series system with an Apollo C18 column (250 mm × 4.6 mm, 5 μm; Grace Davison). GC analysis was performed using an Agilent 7890A series system with a capillary column, HP-5 (30 m × 0.25 mm, with a 0.25 μm film, Dikma). Preparative HPLC was carried out on a Shimadzu LC-6AD instrument with an SPD-20A detector (Shimadzu Corp., Tokyo, Japan), using a YMC-Pack ODS-A column (250 mm × 20 mm, 5 μm; YMC Corp., Kyoto, Japan). Column chromatography was performed with macroporous resin (Diaion HP-20, Mitsubishi Chemical Corp., Tokyo, Japan), Rp-18 (50 μm, YMC Corp.), Sephadex LH-20 (Pharmacia Fine Chemicals, Uppsala, Sweden).Plant material
The rhizome of P. cuspidatum was purchased from Beijing Pu Sheng Lin Pharmaceutical Co., Ltd. in Beijing, People's Republic of China, in Feb 2010. The plant material was identified by L. Ma (Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, P. R. China). A voucher specimen (ID number: ID-S-2593) was deposited at the Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, P. R. China.Extraction and isolation
Powdered P. cuspidatum (20 kg) was extracted with 80% EtOH under reflux (3 × 2 h). The EtOH extract was concentrated under reduced pressure to give a residue (4.2 kg), which was suspended in H2O (4 L) and partitioned with, consecutively, petroleum ether (PE) (3 × 4 L), EtOAc (3 × 4 L), and n-BuOH (3 × 4 L). After evaporation of the n-BuOH solvent under reduced pressure, the extract (1375 g) was suspended in H2O (2 L) and subjected to column chromatography over macroporous resin, eluting successively with H2O, 30% EtOH, 50% EtOH, 70% EtOH, and 95% EtOH (30 L each). The 30% EtOH was removed under reduced pressure and the fraction (86.4 g) was subjected to chromatography over Sephadex LH-20 with H2O–MeOH in gradient as the mobile phase to yield 25 fractions (Fr. A–Fr. Y) on the basis of HPLC-DAD analysis.Fr. B was subjected to reversed-phase preparative HPLC, using MeOH–H2O (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76) as the mobile phase, to give 11 (25 mg) and 12 (22 mg). Compound 10 (12 mg) was obtained from Fr. C by recrystallization. Fr. D was purified by preparative RP-HPLC using MeOH–H2O (28
76) as the mobile phase, to give 11 (25 mg) and 12 (22 mg). Compound 10 (12 mg) was obtained from Fr. C by recrystallization. Fr. D was purified by preparative RP-HPLC using MeOH–H2O (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72) as the mobile phase to yield 14 (53 mg). Fr. E was purified by preparative RP-HPLC using MeOH–H2O (31
72) as the mobile phase to yield 14 (53 mg). Fr. E was purified by preparative RP-HPLC using MeOH–H2O (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69) as the mobile phase to yield 13 (13 mg). Fr. I was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
69) as the mobile phase to yield 13 (13 mg). Fr. I was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 20 fractions (Fr. I-1–I-20). Fr. I-13 was further purified using preparative RP-HPLC with MeOH–H2O (50
40) to afford 20 fractions (Fr. I-1–I-20). Fr. I-13 was further purified using preparative RP-HPLC with MeOH–H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as the mobile phase to yield 15 (8 mg). Fr. N was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
50) as the mobile phase to yield 15 (8 mg). Fr. N was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 20 fractions (Fr. N-1–N-20). Fr. N-9 was further purified using preparative RP-HPLC with MeOH–H2O (33
40) to afford 20 fractions (Fr. N-1–N-20). Fr. N-9 was further purified using preparative RP-HPLC with MeOH–H2O (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67) as the mobile phase to yield 6 (11 mg), 7 (10 mg), 3 (10 mg), and 5 (6 mg). Fr. N-10 was further purified using preparative RP-HPLC with MeOH–H2O (40
67) as the mobile phase to yield 6 (11 mg), 7 (10 mg), 3 (10 mg), and 5 (6 mg). Fr. N-10 was further purified using preparative RP-HPLC with MeOH–H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) as the mobile phase to yield 2 (13 mg). Fr. O was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
60) as the mobile phase to yield 2 (13 mg). Fr. O was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 20 fractions (Fr. O-1–O-20). Fr. O-18 was further purified using preparative RP-HPLC with MeOH–H2O (34
40) to afford 20 fractions (Fr. O-1–O-20). Fr. O-18 was further purified using preparative RP-HPLC with MeOH–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66) as the mobile phase to yield 4 (10 mg). Fr. Q was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
66) as the mobile phase to yield 4 (10 mg). Fr. Q was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 15 fractions (Fr. Q-1–Q-15). Fr. Q-12 was further purified using preparative RP-HPLC with MeOH–H2O (19
40) to afford 15 fractions (Fr. Q-1–Q-15). Fr. Q-12 was further purified using preparative RP-HPLC with MeOH–H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81) as the mobile phase to yield 9 (11 mg). Fr. R was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
81) as the mobile phase to yield 9 (11 mg). Fr. R was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 20 fractions (Fr. R-1–R-20). Fr. R-11 was further purified using preparative RP-HPLC with MeOH–H2O (33
40) to afford 20 fractions (Fr. R-1–R-20). Fr. R-11 was further purified using preparative RP-HPLC with MeOH–H2O (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67) as the mobile phase to yield 8 (10 mg). Fr. U was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20
67) as the mobile phase to yield 8 (10 mg). Fr. U was subjected to Sephadex LH-20 column and eluted with MeOH–H2O (from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 to 60
80 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford 30 fractions (Fr. U-1–U-30). Fr. U-25 was further purified using preparative RP-HPLC with MeOH–H2O (38
40) to afford 30 fractions (Fr. U-1–U-30). Fr. U-25 was further purified using preparative RP-HPLC with MeOH–H2O (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62) as the mobile phase to yield 1 (24 mg).
62) as the mobile phase to yield 1 (24 mg).
Structure characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 280 (0.26) nm; ECD (MeOH) λmax (Δε) 285 (−3.41), 222 (−5.38) nm; IR (KBr) νmax: 3372, 1613, 1513, 1450 cm−1; HRESIMS m/z 847.2094 [M − H]− (calcd for C42H40O19, 847.2091); 1H and 13C NMR data, see Tables 1 and 2.
ε) 280 (0.26) nm; ECD (MeOH) λmax (Δε) 285 (−3.41), 222 (−5.38) nm; IR (KBr) νmax: 3372, 1613, 1513, 1450 cm−1; HRESIMS m/z 847.2094 [M − H]− (calcd for C42H40O19, 847.2091); 1H and 13C NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 283 (0.21), 316 (0.14) nm; ECD (MeOH) λmax (Δε) 276 (+6.54), 234 (−27.99) nm; IR (KBr) νmax: 3328, 1603, 1514, 1453 cm−1; HRESIMS m/z 819.2478 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.
ε) 283 (0.21), 316 (0.14) nm; ECD (MeOH) λmax (Δε) 276 (+6.54), 234 (−27.99) nm; IR (KBr) νmax: 3328, 1603, 1514, 1453 cm−1; HRESIMS m/z 819.2478 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 283 (0.21), 323 (0.12) nm; ECD (MeOH) λmax (Δε) 298 (−3.16), 238 (−3.33) nm; IR (KBr) νmax: 3365, 1604, 1511, 1453 cm−1; HRESIMS m/z 819.2464 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.
ε) 283 (0.21), 323 (0.12) nm; ECD (MeOH) λmax (Δε) 298 (−3.16), 238 (−3.33) nm; IR (KBr) νmax: 3365, 1604, 1511, 1453 cm−1; HRESIMS m/z 819.2464 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 284 (0.21), 320 (0.13) nm; ECD (MeOH) λmax (Δε) 280 (−2.93), 214 (−19.37) nm; IR (KBr) νmax: 3328, 1574, 1514, 1416 cm−1; HRESIMS m/z 819.2463 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.
ε) 284 (0.21), 320 (0.13) nm; ECD (MeOH) λmax (Δε) 280 (−2.93), 214 (−19.37) nm; IR (KBr) νmax: 3328, 1574, 1514, 1416 cm−1; HRESIMS m/z 819.2463 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 84 (0.21), 321 (0.13) nm; ECD (MeOH) λmax (Δε) 279 (+0.31), 248 (−1.34), 230 (+6.32) nm; IR (KBr) νmax: 3340, 1604, 1513, 1452 cm−1; HRESIMS m/z 819.2468 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.
ε) 84 (0.21), 321 (0.13) nm; ECD (MeOH) λmax (Δε) 279 (+0.31), 248 (−1.34), 230 (+6.32) nm; IR (KBr) νmax: 3340, 1604, 1513, 1452 cm−1; HRESIMS m/z 819.2468 [M + Na]+ (calcd for C40H44O17Na, 819.2471); 1H and 13C NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 307 (0.43), 321 (0.42) nm; IR (KBr) νmax: 3348, 1592, 1513, 1445 cm−1; HRESIMS m/z 553.1920 [M + H]+ (calcd for C26H33O13, 553.1916); 1H and 13C NMR data, see Tables 1 and 3.
ε) 307 (0.43), 321 (0.42) nm; IR (KBr) νmax: 3348, 1592, 1513, 1445 cm−1; HRESIMS m/z 553.1920 [M + H]+ (calcd for C26H33O13, 553.1916); 1H and 13C NMR data, see Tables 1 and 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 306 (0.43), 321 (0.42) nm; IR (KBr) νmax: 3317, 1603, 1514, 1449 cm−1; HRESIMS m/z 553.1911 [M + H]+ (calcd for C26H33O13, 553.1916); 1H and 13C NMR data, see Tables 1 and 3.
ε) 306 (0.43), 321 (0.42) nm; IR (KBr) νmax: 3317, 1603, 1514, 1449 cm−1; HRESIMS m/z 553.1911 [M + H]+ (calcd for C26H33O13, 553.1916); 1H and 13C NMR data, see Tables 1 and 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 296 (0.51), 323 (0.41) nm; IR (KBr) νmax: 3297, 1701, 1603, 1514, 1450, 1218, 1047 cm−1; HRESIMS m/z 621.0929 [M − H]− (calcd for C27H25O15S, 621.0920); 1H and 13C NMR data, see Tables 1 and 3.
ε) 296 (0.51), 323 (0.41) nm; IR (KBr) νmax: 3297, 1701, 1603, 1514, 1450, 1218, 1047 cm−1; HRESIMS m/z 621.0929 [M − H]− (calcd for C27H25O15S, 621.0920); 1H and 13C NMR data, see Tables 1 and 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 280 (0.40) nm; ECD (MeOH) λmax (Δε) 285 (−3.41), 236 (−5.38) nm; IR (KBr) νmax: 3363, 1698, 1610, 1528, 1504, 1453 cm−1; HRESIMS m/z 627.1316 [M + Na]+ (calcd for C28H28O15Na, 627.1320); 1H and 13C NMR data, see Tables 1 and 3.
ε) 280 (0.40) nm; ECD (MeOH) λmax (Δε) 285 (−3.41), 236 (−5.38) nm; IR (KBr) νmax: 3363, 1698, 1610, 1528, 1504, 1453 cm−1; HRESIMS m/z 627.1316 [M + Na]+ (calcd for C28H28O15Na, 627.1320); 1H and 13C NMR data, see Tables 1 and 3.Cellulose hydrolysis of 1–5
A solution of 1 (2 mg) in 2 mL 0.1 M HOAc–NaOAc buffer (pH 4.5) was incubated at 40 °C with cellulose (2 mg) for 5 h. The reaction was monitored by HPLC-DAD analysis. After cooling, the reaction mixture was extracted with EtOAc (3 × 5 mL). The aqueous layer was evaporated under vacuum to furnish a crude sugar fraction. Hydrolysis of 2–5 was performed according to the procedure described for 1.Acid hydrolysis of 6–9
Compound 6 (2 mg) was dissolved in 0.5 M HCl–H2O (2 mL) and refluxed for 2 h. After cooling, the reaction mixture was extracted with EtOAc (3 × 5 mL). The aqueous layer was evaporated under vacuum to furnish a crude sugar fraction. Hydrolysis of 7–9 was performed according to the procedure described for 6.Gas chromatographic (GC) analysis of glucose
The crude sugar fraction was dissolved in anhydrous pyridine (1 mL), to which 2 mg of L-cysteine methyl ester hydrochloride was added. The mixture was stirred at 60 °C for 2 h. After evaporation under reduced pressure, 0.2 mL of N-trimethylsilylimidazole was added, and the mixture was kept at 60 °C for another 2 h. The reaction mixture was partitioned between n-hexane and H2O (2 mL each), and the n-hexane extract was analysed by GC under the following conditions: capillary column, HP-5 (30 m × 0.25 mm, with a 0.25 μm film, Dikma); detection, FID; detector temperature, 280 °C; injection temperature, 250 °C; initial temperature 160 °C, then raised to 280 at 5 °C min−1, final temperature maintained for 10 min; carrier, N2 gas. D-Glucose was confirmed by comparison of the retention time of its derivative with the authentic sugar derivatized in a similar way, which exhibited a retention time of 19.03 min.Inhibitory activity of α-glucosidase
The inhibitory activity of compounds 1–15 on α-glucosidase was determined spectrophotometrically on a 96-well microplate reader. In total, 20 μL of 0.2 U mL−1 α-glucosidase was premixed with 10 μL of compounds at various concentrations in 50 μL of 100 mM phosphate buffer (pH 7.0) at 37 °C for 5 min. Then, 20 μL of 2.5 mM substrate p-nitrophenyl-α-D-glucopyranoside was added to the mixture to initiate the reaction. The reaction was incubated at 37 °C for 15 min and stopped by the addition of 50 μL of 0.4 M Na2CO3. α-Glucosidase activity was determined by measuring the release of p-nitrophenol from p-nitrophenyl-α-D-glucopyranoside at 400 nm. The sample contained the mixture and the test compound, while the control consisted of the mixture, including the solvent, but without test compound. The sample blank contained the mixture, including the test compound, but without α-glucosidase. Finally, the control blank was the mixture, including the solvent, but without α-glucosidase.The inhibition (%) of sample on α-glucosidase was calculated by the following formula:
| Inhibition (%) = [(A(sample) − A(sample blank))/(A(control) − A(control blank))] × 100 |
Conclusions
Herein, we reported the isolation and structure elucidation of nine new compounds from the rhizome of P. cuspidatum, including an unusual adduct of ECG fused to piceid through a carbon–carbon bond (1), four new dimeric stilbene glycosides (2–5), three new stilbene glucosides (6–8), and one new flavan glucoside (9). In order to determine the absolute configurations for the chiral carbons, the Rh2(OCOCF3)4-induced CD assay was combined with the characteristic coupling constant and ECD spectrum analysis. Further investigation of in vitro bioactivities of 1–15 revealed that compound 8 showed significantly stronger inhibition activity than the positive control acarbose. This result indicated that compound 8 might be useful as a glucosidase inhibitor agent for the treatment of type 2 diabetes.Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81274066) and Natural Science Foundation of Beijing Municipality (No. 7112094).References
- W. Peng, R. Qin, X. L. Li and H. Zhou, J. Ethnopharmacol., 2013, 148, 729–745 CrossRef CAS PubMed.
- H. Zhang, C. Li, S. T. Kwok, Q. W. Zhang and S. W. Chan, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 208349 Search PubMed.
- C. C. Lee, Y. T. Chen, C. C. Chiu, W. T. Liao, Y. C. Liu and H. M. David Wang, J. Biosci. Bioeng., 2015, 119, 464–469 CrossRef CAS PubMed.
- J. S. Chang, H. W. Liu, K. C. Wang, M. C. Chen, L. C. Chiang, Y. C. Hua and C. C. Lin, Antiviral Res., 2005, 66, 29–34 CrossRef CAS PubMed.
- B. Shan, Y. Z. Cai, J. D. Brooks and H. Corke, Food Chem., 2008, 109, 530–537 CrossRef CAS.
- F. S. Chueh, J. J. Lin, J. P. Lin, F. S. Yu, J. H. Lin, Y. S. Ma, Y. P. Huang, J. C. Lien and J. G. Chung, In Vivo, 2015, 29, 255–261 Search PubMed.
- T. Liu, H. Jin, Q. R. Sun, J. H. Xu and H. T. Hu, Brain Res., 2010, 1347, 149–160 CrossRef CAS PubMed.
- H. Matsuda, H. Shimoda, T. Morikawa and M. Yoshikawa, Bioorg. Med. Chem. Lett., 2001, 11, 1839–1842 CrossRef CAS PubMed.
- K. Xiao, L. J. Xuan, Y. M. Xu and D. L. Bai, J. Nat. Prod., 2000, 63, 1373–1376 CrossRef CAS.
- G. S. Jayatilake, H. Jayasuriya, E. S. Lee, N. M. Koonchanok, R. L. Geahlen, C. L. Ashendel, J. L. McLaughlin and C. J. Chang, J. Nat. Prod., 1993, 56, 1805–1810 CrossRef CAS.
- K. Xiao, L. J. Xuan, Y. M. Xu, D. L. Bai and D. X. Zhong, Chem. Pharm. Bull., 2002, 50, 605–608 CrossRef CAS PubMed.
- Z. Y. Yang, Q. Z. Cai, N. Chen, X. M. Zhou and J. L. Hong, RSC Adv., 2016, 6, 12193–12204 RSC.
- F. S. Li, Z. L. Zhan, F. Liu, Y. N. Yang, L. Li, Z. M. Feng, J. S. Jiang and P. C. Zhang, Org. Lett., 2013, 15, 674–677 CrossRef CAS PubMed.
- F. Liu, F. S. Li, Z. M. Feng, Y. N. Yang, J. S. Jiang and P. C. Zhang, Phytochemistry, 2015, 110, 150–159 CrossRef CAS PubMed.
- J. M. van Der Nat, W. G. van der Sluis, L. A. Hart, H. Van Dijk, K. T. D. de Silva and R. P. Labadie, Planta Med., 1991, 57, 65–68 CrossRef CAS PubMed.
- J. Frelek, A. Klimek and P. Ruskowska, Curr. Org. Chem., 2003, 7, 1081–1104 CrossRef CAS.
- K. Xiao, L. J. Xuan, Y. M. Xu, D. L. Bai, D. X. Zhong, H. M. Wu, Z. H. Wang and N. X. Zhang, Eur. J. Org. Chem., 2002, 3, 564–568 CrossRef.
- J. Hu, T. Lin, J. Y. Xu, R. Ding, G. H. Wang, R. C. Shen, Y. W. Zhang and H. F. Chen, Bioorg. Med. Chem. Lett., 2016, 26, 505–511 CrossRef CAS PubMed.
- T. Stark and T. Hofmann, J. Agric. Food Chem., 2006, 54, 9510–9521 CrossRef CAS PubMed.
- Z. Y. Ren, H. Y. Qi and Y. P. Shi, Planta Med., 2008, 74, 859–863 CrossRef CAS PubMed.
- T. Tanaka, Y. Orii, G. I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1993, 41, 1232–1237 CrossRef CAS.
- Y. Dai, X. J. He, G. X. Zhou, H. Kurihara, W. C. Ye and X. S. Yao, J. Asian Nat. Prod. Res., 2008, 10, 111–117 CrossRef CAS PubMed.
- P. Königs, B. Rinker, L. Maus, M. Nieger, J. Rheinheimer and S. R. Waldvogel, J. Nat. Prod., 2010, 73, 2064–2066 CrossRef PubMed.
- C. G. Girol, K. M. Fisch, T. Heinekamp, S. Günther, W. Hüttel, J. Piel, A. A. Brakhage and M. Müller, Angew. Chem., Int. Ed., 2012, 51, 9788–9791 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1D NMR, 2D NMR HRMS, IR, ECD and Rh2(OCOCF3)4 induced CD spectra. See DOI: 10.1039/c6ra11135a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |