Graphene oxide modified metallic lithium electrode and its electrochemical performances in lithium–sulfur full batteries and symmetric lithium–metal coin cells†
Yi-jun Zhang,
Xin-hui Xia,
Xiu-li Wang,
Chang-dong Gu and
Jiang-ping Tu*
State Key Laboratory of Silicon Materials, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: tujp@zju.edu.cn; tujplab@zju.edu.cn
First published on 6th July 2016
Abstract
The growth of dendrites greatly hinders the practical applications of lithium metal batteries. In this work, we adopt an automatic spreading method to coat graphene oxide (GO) layers on metallic lithium foil to form a composite electrode. The GO layers are distributed homogeneously and show low roughness on the metallic lithium foil. The electrochemical performance of GO modified Li (GO/Li) electrodes have been thoroughly studied. The GO/Li electrode displays enhanced electrochemical performances in a lithium–sulfur full battery, and better cycling stability in symmetric lithium–metal coin cells than the unmodified pure Li electrode. In addition, the growth of dendritic Li is successfully suppressed in the GO/Li electrode.
1. Introduction
Rechargeable lithium-based batteries, such as Li–sulfur, Li–air and lithium ion batteries, have been extensively studied for electrochemical energy storage because of their high working voltage, large energy density, and long cycling life.1–7 However, all these batteries use Li metal as the anode, and encounter great challenges as the formation of Li dendrites during cycling causes great safety hazards.8 Constructing a stable and effective solid electrolyte interphase (SEI) is one of the most effective strategies to inhibit the dendrite growth and thus to achieve a superior cycling performance.9–11 For example, the SEI on the graphite anode can be extremely stable during several thousands of cycles in the carbonate electrolyte, the Li-ion batteries with the graphite anode have been successfully commercialized.12,13 However, the SEI layer of Li metal is usually unstable and is not yet well understood. Compared with volume changes of graphite (∼10%) and silicon anodes (∼300%), respectively, the relative volumetric change of Li metal is virtually infinite, because Li metal is “hostless”. The huge volume change requires the SEI layer with high elastic modulus.14 For an ideal SEI, it should possess several characteristics, such as high Li ionic conductivity, proper thickness with compact structure, and high elastic strength to mechanically suppress the burst of Li dendrites.15–17 However, the SEI layer of lithium metal batteries is unstable in some extreme operation conditions, such as extreme high/low temperature and high cycling rates. The formed SEI either grows much thicker or becomes non-protective, which induces rapid performance degradation. Up to now, the SEI modification methods of Li metal are classified as follows: (1) changing electrolyte component to stabilize SEI, such as adding additives into the electrolyte;18–27 (2) treating the Li metal with chosen chemicals to coat an ex situ formed protective layer (or ‘artificial’ SEI layer) on the Li metal;14,28–38 (3) redesigning the Li anode structure to postpone the growth of Li dendrites.8,39 It is very difficult to achieve sufficient passivation between the Li metal anodes and the electrolyte through the 1st method, as indicated by Aurbach.40 While concerning to redesign the Li anode structure (the 3rd method), it is not convenient in practical application. The 2nd approach is to make a pre-formed protective film/layer with high Li ion conductivity, such as carbon films,28,29,33–35 and lithium nitrogenous compounds.31,32 The protective layer can not only prevent Li metal anodes from direct contacting with the electrolyte, but also improve the uniformity of SEI film and suppress the growth of dendritic Li.As a two-dimensional crystal of sp2 conjugated carbon atoms, graphene sheet possesses high Li ion conductivity and excellent mechanical strength,41 which makes it a promising candidate as the protective layer for Li metal anode. According to the previously reports,35,42 the GO sheets will orderly stack layer by layer after suction filtration and can inhibit the formation of dendritic lithium essentially when composite with lithium. Unsatisfactory, the filtration of GO sheets is demanding and time-consuming. In order to simplify the preparation process, an automatic spreading method is used to covering GO film on lithium electrode. Theoretically, the liquid solution will spread evenly on the substrate under the liquid tension, and the GO sheets will flat and descend. After the organic solvent fully volatilized, there will be only GO film covered on the basal surface and the thickness of GO film can be controlled through adjusting the concentration of solution. As the molecules of GO is chemical polar, therefore can be dissolved in polar solvent according to the principle of polarity mutually soluble. Unfortunately, most of the polar solvent is chemically activity to metal lithium, which made the choice of the solvent become a problem. In order to choose the appropriate organic solvent, the ethanol, acetonitrile, ether and DMC are tested and the DMC is determined ultimately. In this present work, automatic spreading method which use DMC as the solvent is used to fabricate GO/Li electrode, and its electrochemical properties is investigated in detail. Enhanced cycling capacity and cycling life are obtained for the GO/Li electrode due to the rational modification of GO on Li metal.
2. Experimental
2.1 Material fabrication
GO was prepared by a modified Hummers' method, which was similar to our previous reports.43–46 To prepare the graphene solution, 2 mg GO powder was dispersed with 10 mL high purity anhydrous DMC, and then the mixture was vigorous stirring and treated by ultrasonication. The GO powder was dispersed homogeneously and a transparent light brown solution can be obtained. The lithium foils were pasted on copper sheets with double-side adhesive, the thickness of Li foil was 0.3 mm while the thickness of Cu sheet was 0.05 mm. Then, the GO/DMC solution was dropped on the Li/Cu composite belt. Under the action of liquid tension, the GO sheets would coat on lithium foil as the GO/DMC solution spreading. The GO/Li electrodes were fabricated by punching the graphene modified Li/Cu composite foil into 15 mm wafer. The area with double-side adhesive must be avoided when punching the electrode wafer. The Cu sheet was used as the current collector during the following electrochemical cycling. The schematic diagram is shown in Fig. 1.2.2 Characterization
The microtopography of GO/Li electrodes was characterized by scanning electron microscope (SEM, Hitachi S-4800 equipped with GENENIS 4000 EDAX detector) and transmission electron microscope (TEM, JEOL JEM-2100). The structure and composition were characterized by X-ray diffraction (XRD, Rigaku D/Max-3B) and Raman spectra (JobinYvon Labor Raman HR-800). The bonded structures of pure Li and GO/Li electrodes after cycling were examined by an X-ray photoelectron spectroscopy (XPS) using an ESCAL 220i-XL electron spectrometer, operating with a monochromated Al-KX-ray radiation source in a base pressure of 10−7 Pa. Before characterization, the modified Li foil was sealed with a polyimide film in order to prevent undesirable reactions in air.Lithium–sulfur full batteries and symmetric lithium–metal coin cells (CR2025 type) were assembled in a glove box filled with high-purity argon. The lithium–sulfur batteries were prepared for the characterization of electrochemical performance. The symmetric lithium–metal coin cells were used for research the stability of lithium electrodeposition. A polypropylene micro-porous film (Celgard 2300) was used as the separators. The electrolyte of lithium–sulfur batteries was 1 M bis (trifluoromethane) sulfonamide lithium salt (LiTFSI) in a mixed solvent of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, including 1 wt% LiNO3 as an electrolyte additive. While a solution of 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1
1, including 1 wt% LiNO3 as an electrolyte additive. While a solution of 1 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) was employed as the electrolyte for symmetric lithium–metal coin cells. The sulfur cathode is modified by carbon black (CCB) and Co(OH)2 as previously reports.47,48 The Co(OH)2@S/CCB composite was synthesized by a simple hydrothermal method. In a typical experiment, 0.05 M Co(NO3)2·6(H2O) and 0.025 M C6H12N4 were dissolved in deionized water, followed by magnetic stirring for 30 min, then 0.5 g S/C composite and 0.01 g polyvinyl pyrrolidone (PVP) were added and kept stirring and ultrasonicating to form a stable aqueous dispersion. Afterwards, the mixture was transferred to a 100 mL autoclave and heated at 90 °C for 2 h. After cooling to room temperature, the product was sequentially washed and filtered with deionized water several times, and dried in a vacuum oven at 60 °C overnight. The average S mass loading of the cathode is about 1.5 mg cm−2.
1 in volume) was employed as the electrolyte for symmetric lithium–metal coin cells. The sulfur cathode is modified by carbon black (CCB) and Co(OH)2 as previously reports.47,48 The Co(OH)2@S/CCB composite was synthesized by a simple hydrothermal method. In a typical experiment, 0.05 M Co(NO3)2·6(H2O) and 0.025 M C6H12N4 were dissolved in deionized water, followed by magnetic stirring for 30 min, then 0.5 g S/C composite and 0.01 g polyvinyl pyrrolidone (PVP) were added and kept stirring and ultrasonicating to form a stable aqueous dispersion. Afterwards, the mixture was transferred to a 100 mL autoclave and heated at 90 °C for 2 h. After cooling to room temperature, the product was sequentially washed and filtered with deionized water several times, and dried in a vacuum oven at 60 °C overnight. The average S mass loading of the cathode is about 1.5 mg cm−2.
The galvanostatic charge–discharge tests were carried out on LAND battery test system. Cyclic voltammogram (CV) tests were carried out using the CHI660C electrochemical workstation in a potential range of 1.5–3.0 V (vs. Li/Li+) at a scan rate of 1 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were performed on the CHI660C over a frequency range of 100 kHz to 10 mHz and the amplitude was set to 10 mV. After the electrochemical tests, the working electrodes were removed from the cells with the battery removal machine (Kejing, MSK-110D).
3. Results and discussion
In our assumption, the GO sheets will disperse in the solvent evenly at the beginning, and flat on the substrate as the solvent spread out on the lithium foil under the liquid tension. After the solvent volatilized, the GO sheets will flat on the lithium foil layer by layer orderly, the schematic diagram can be seen in Fig. 2a. In order to choose the appropriate organic solvent, the ethanol, acetonitrile, ether and DMC are dropped on the surface of lithium foils. Fig. 2b–e displays the digital photos of lithium foils contacted with different solvent. The reaction between the surface of lithium foils and ethanol, acetonitrile and ether can be observed clearly, while there is no visible change of the lithium surface contacted with DMC. Then the SEM analysis is applied to observe the microtopography of lithium surface contacted with different solvent, as shown in Fig. 2f–k. The surface of Li foils contacted with ethanol and acetonitrile are full of bubbles at the micro scale, results from the active group in the solvent molecules. The –OH group in alcohol molecule and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group in acetonitrile molecule will react with Li and the liberation of gas will lead to the continuous stomatal morphology. The surface of Li foils contacted with ether turns black on a large scale and displays a rimous morphology at the micro scale. It is suggested that there will be an oxidation reaction between the ether and the Li due to the C
N group in acetonitrile molecule will react with Li and the liberation of gas will lead to the continuous stomatal morphology. The surface of Li foils contacted with ether turns black on a large scale and displays a rimous morphology at the micro scale. It is suggested that there will be an oxidation reaction between the ether and the Li due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in ether molecule. By contrast, there is no difference between the DMC contacted Li foil (Fig. 2k) and pure Li foil (Fig. 2l) in both large and micro scale, which means that DMC is chemical inertness to metallic Li. So DMC is chosen to be the solvent of GO finally.
O group in ether molecule. By contrast, there is no difference between the DMC contacted Li foil (Fig. 2k) and pure Li foil (Fig. 2l) in both large and micro scale, which means that DMC is chemical inertness to metallic Li. So DMC is chosen to be the solvent of GO finally.
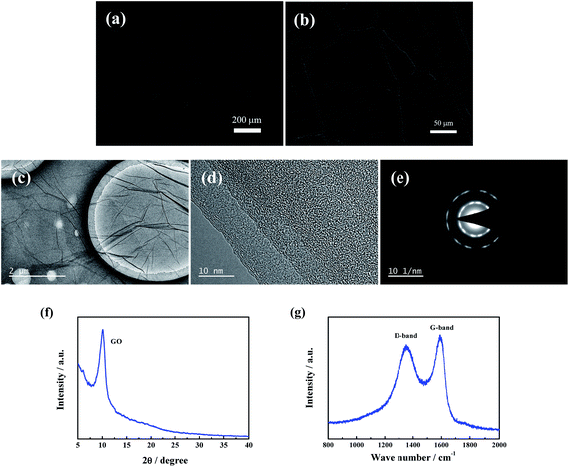
The microtopography and structure analysis of the GO layer on lithium foil are displayed in Fig. 3. The surface topography of Li/GO electrode in different amplification is shown in Fig. 3a and b. The pure Li electrode shows a flat surface with metallic luster (Fig. 2l). By contrast, the surface morphology of Li foil modified by GO layers is similar to the vast plain, and the joint between the pieces of GO sheets present a perfect connection. It can be drawn that the GO layers spread on lithium surface uniformly and continuously. The TEM image, HRTEM image and SAED pattern of GO layers spread on lithium foil can be seen in Fig. 3c–e. The GO sheets flat layer by layer with a slight fold (Fig. 3c), and the typical fingerprint crystal morphology of GO can be observed clearly in the HRTEM image (Fig. 3d). The XRD patterns and Raman spectrum of GO layers are shown in Fig. 3f and g, presenting a typical curve shape of GO material without any other peak, which means that the DMC solvent has been volatilized completely and there is no adverse reaction happened between lithium and the solvent.
Fig. 4 shows the electrochemical performances of pure Li and GO/Li electrodes in Li–S batteries. The CV curves of pure Li–S and GO/Li–S full batteries are shown in Fig. 4a. Both batteries show two main reduction peaks around 2.3 V and 2.0 V, corresponding to the generation of long-chain soluble lithium polysulfide (Li2Sn, 4 ≤ n < 8) and short-chain insoluble lithium polysulfide (Li2S2 or Li2S), respectively. And there is only one peak located at 2.4 V in the oxidation process, which can be ascribed to the conversion of Li2S2 and Li2S into the polysulfide, matching well with the redox process of the lithium sulfur battery.49–51 The CV profiles of Li–S battery show higher current density and reaction area than the GO/Li–S full battery, which can be ascribed to the poor conductive property of GO layers. The impedances of Li–S and GO/Li–S batteries before and after the first cycle are presented in Fig. S1 (see ESI†). It is obvious that the charge transfer resistance of GO/Li–S battery before the first cycle is much larger than that after the first cycle, indicating the poor conductivity of GO layers. Nevertheless, the charge transfer resistance of GO/Li–S battery after the first cycle reduced significantly, even smaller than that of Li–S battery, suggesting that the poor conductivity of GO layer won't lead to the poor electrochemical performance of GO/Li–S battery. Fig. 4b displays the charge/discharge profiles of pure Li–S and GO/Li–S batteries at 0.1C (167.5 mA g−1 – S) after the first cycle. The initial charge and discharge capacity of pure Li–S battery is 860 mA h g−1 and 840 mA h g−1, while that of GO/Li–S battery is 1043 mA h g−1 and 1025 mA h g−1, respectively. The first charge and discharge efficiency of GO/Li–S battery is about 98.3%, is a little bit higher than the initial efficiency (97.7%) of pure Li–S battery. Compared with the previous results,52,53 the capacity and stability of the Li–S battery has been improved obviously. For the further research, the galvanostatic charge/discharge and coulombic efficiency are tested, as shown in Fig. 4c. The capacity of pure Li–S battery decreases from 840 mA h g−1 to 487 mA h g−1 after 200 cycles. On the contrary, benefiting from the GO layers protection, the GO/Li–S battery exhibits a better cycling capacity. The capacity is still maintained at 707 mA h g−1 after 200 cycles. Apparently, the GO/Li–S full battery has better electrochemical performance. The Nyquist plots of pure Li–S and GO/Li–S full batteries after 10 cycles can be seen in Fig. 4d. All of the plots are mainly composed of a small intercept at high frequency, an arc at high to medium frequency and a linear part in low frequency. The arc in high frequency region represents the charge transfer resistance Rct and the electrical double-layer capacitance. The straight line in low frequency region reflects the conductivity of Li+. Compared of the curve shape of pure Li–S and GO/Li–S full batteries, it is demonstrated that the GO/Li–S battery presents a smaller charge transfer resistance and a larger Li ion conductivity, which can be attributed to the protection of GO layers. In details, some side reactions occur on the surface of Li anode during the initial cycling process, and the reaction products accumulated on the surface of anode will hinder the transfer of electrons and Li ions. As the GO layers on Li surface can reduce the side reaction, there will be less side reaction products accumulated on the surface of GO/Li anode, which lead to the small charge transfer resistance and high Li ion conductivity.
Voltage versus time profiles for pure Li and GO/Li electrodes in symmetric lithium cells are shown in Fig. 5. Each half cycle lasts for 3 h at 0.4 mA cm−2 and 0.5 mA cm−2, respectively. Three-hour interval of strip/plate is chosen to allow enough lithium to transport between the electrodes, hence the voltage change could be fully observed.25–27,54 The voltage versus time profiles for pure Li–Li symmetric lithium cell at 0.4 mA cm−2 shows an erratic voltage response (Fig. 5a), while the GO/Li–Li symmetric lithium cell at the same current density maintains stably over 600 h. When the current density is increased to 0.5 mA cm−2, the voltage versus time profiles for both pure Li–Li and GO/Li–Li symmetric lithium cells vibrate more violently. By contrast, the voltage versus time profiles for pure Li–Li symmetric lithium cell up and down more irregularly. The haphazard changes in the profiles indicate the poor stability of the cell, which can be ascribed to the dendrite formation on the surface of pure Li electrodes. The good stability of GO/Li–Li symmetric lithium cell can be attributed to the protective effect of GO film.
The digital photos and SEM images of pure Li and GO/Li electrodes after 200 h cycling process in symmetric lithium cell system are shown in Fig. 6. The physical photo of pure Li electrodes shows that the surface of pure Li electrode is rugged and local reacted (Fig. 6a). The dendrite clusters of lithium can be observed clearly after magnified in 5 hundred times (Fig. 6b), while the SEM image under 5 thousand times displays obvious sharp granule morphology (Fig. 6c). After modified by GO layers, the surface reaction of Li electrode is uniform and moderate, which can be obtained from the photo of GO/Li electrode (Fig. 6d). Fig. 6e and f are the SEM images of GO/Li electrodes under 5 hundred times and 5 thousand times, both of which present a flat mossy-like morphology. It can be suggested that the GO layers can help suppress the formation of dendritic Li efficiently.
 | ||
| Fig. 6 The digital photos and SEM images of pure Li (a), (b), (c); and GO/Li electrodes (d), (e), (f) after 200 h cycling in symmetric lithium cell. | ||
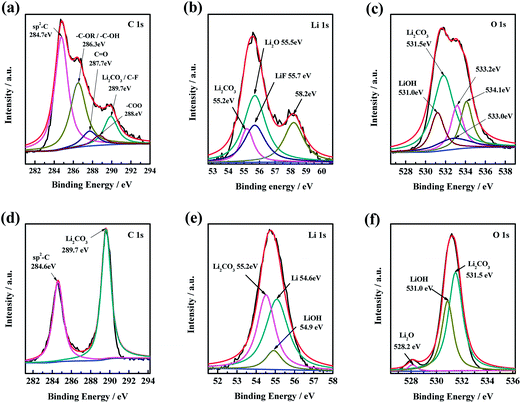
Fig. 7 shows the C 1s, Li 1s and O 1s XPS spectra of pure Li and GO/Li electrodes after 200 h cycling process in symmetric lithium cells. Fig. 7a–c shows the C 1s, Li 1s and O 1s XPS spectra of pure Li electrode after 200 h cycling, respectively. According to the NIST X-ray Photoelectron Spectroscopy Database, the peaks at 284.7 eV in C 1s XPS spectra can be assigned to the graphite band (G-band, sp2 bonding) (Fig. 7a). In addition, the C–O bonding, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding, –C–OR/–C–OH bonding and Li2CO3/C–F bonding are also found at binding energy of 288, 287.7, 286.3 and 289.7 eV, respectively. While in the Li 1s XPS spectra of the pure Li electrode after 200 h cycles (Fig. 7b), there is an unknown peaks at 58.2 eV existed except for the peak of LiO2, LiF and Li2CO3. The peak at 58.2 eV of Li 1s spectrum in Fig. 7b and the peaks at 533.2 eV, 534.1 eV and 533.0 eV of O 1s spectrum in Fig. 7c can be attributed to the complex organic compounds generated by Li and the electrolyte during charge–discharge process. By contrast, the constituent of the surface of GO/Li electrode after 200 h cycles is not that complicated. There is only sp2-C, Li, Li2O, LiOH and Li2CO3 can be observed in Fig. 7d–f. The unknown composition of the pure Li electrode can be ascribed to the side reaction between metallic Li and the electrolyte. It is obvious that the side reaction between the lithium electrode and the electrolyte can be inhibited effectively after modified by GO layers.
O bonding, –C–OR/–C–OH bonding and Li2CO3/C–F bonding are also found at binding energy of 288, 287.7, 286.3 and 289.7 eV, respectively. While in the Li 1s XPS spectra of the pure Li electrode after 200 h cycles (Fig. 7b), there is an unknown peaks at 58.2 eV existed except for the peak of LiO2, LiF and Li2CO3. The peak at 58.2 eV of Li 1s spectrum in Fig. 7b and the peaks at 533.2 eV, 534.1 eV and 533.0 eV of O 1s spectrum in Fig. 7c can be attributed to the complex organic compounds generated by Li and the electrolyte during charge–discharge process. By contrast, the constituent of the surface of GO/Li electrode after 200 h cycles is not that complicated. There is only sp2-C, Li, Li2O, LiOH and Li2CO3 can be observed in Fig. 7d–f. The unknown composition of the pure Li electrode can be ascribed to the side reaction between metallic Li and the electrolyte. It is obvious that the side reaction between the lithium electrode and the electrolyte can be inhibited effectively after modified by GO layers.
4. Conclusions
In summary, the GO layers have been successfully fabricated on the surface of lithium metal by a facile automatic spreading method. According to the electrochemical analysis, the GO modified Li electrode can improve the performance of Li–S battery effectively. In symmetric lithium–metal coin cells, the GO/Li electrode displays better stability than the unmodified Li electrode, which can be ascribed to the protection of GO layers. The suppression of dendritic Li has been realized by our GO coating strategy.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51271167) and the Program for Innovative Research Team in University of Ministry of Education of China (IRT13037).References
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- J. C. Guo, Y. H. Xu and C. S. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed.
- S. Lim, R. Lilly Thankamony, T. Yim, H. Chu, Y. J. Kim, J. Mun and T. H. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 1401–1405 CAS.
- L. B. Xing, K. Xi, Q. Y. Li, Z. Su, C. Lai, X. S. Zhao and R. V. Kumar, J. Power Sources, 2016, 303, 22–28 CrossRef CAS.
- X. B. Yang, W. Zhu, G. B. Cao and X. D. Zhao, RSC Adv., 2015, 5, 93926–93936 RSC.
- D. H. Wang, X. H. Xia, D. Xie, X. Q. Niu, X. Ge, C. D. Gu, X. L. Wang and J. P. Tu, J. Power Sources, 2015, 299, 293–300 CrossRef CAS.
- X. Y. Zhao, J. P. Tu, Y. Lu, J. B. Cai, Y. J. Zhang, X. L. Wang and C. D. Gu, Electrochim. Acta, 2013, 113, 256–262 CrossRef CAS.
- C. P. Yang, Y. X. Yin, S. F. Zhang, N. W. Li and Y. G. Guo, Nat. Commun., 2015, 6 DOI:10.1038/ncomms9058.
- D. Aurbach and A. Zaban, J. Electroanal. Chem., 1995, 393, 43–53 CrossRef.
- D. Aurbach and A. Zaban, J. Electrochem. Soc., 1995, 142, L108–L111 CrossRef CAS.
- D. Aurbach, A. Zaban, Y. Gofer, O. Abramson and M. Benzion, J. Electrochem. Soc., 1995, 142, 687–696 CrossRef CAS.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacin, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS PubMed.
- B. Scrosati, J. Hassoun and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 CAS.
- G. Y. Zheng, S. W. Lee, Z. Liang, H. W. Lee, K. Yan, H. B. Yao, H. T. Wang, W. Y. Li, S. Chu and Y. Cui, Nat. Nanotechnol., 2014, 9, 618–623 CrossRef CAS PubMed.
- F. Ding, W. Xu, X. L. Chen, J. Zhang, Y. Y. Shao, M. H. Engelhard, Y. Zhang, T. A. Blake, G. L. Graff, X. J. Liu and J. G. Zhang, J. Phys. Chem. C, 2014, 118, 4043–4049 CAS.
- F. Ding, W. Xu, G. L. Graff, J. Zhang, M. L. Sushko, X. L. Chen, Y. Y. Shao, M. H. Engelhard, Z. M. Nie, J. Xiao, X. J. Liu, P. V. Sushko, J. Liu and J. G. Zhang, J. Am. Chem. Soc., 2013, 135, 4450–4456 CrossRef CAS PubMed.
- X. B. Cheng, R. Zhang, C. Z. Zhao, F. Wei, J. G. Zhang and Q. Zhang, Adv. Sci., 2016, 3, 15002013 Search PubMed.
- F. Croce, G. Appetecchi, L. Persi and B. Scrosati, Nature, 1998, 394, 456–458 CrossRef CAS.
- K. Kanamura, H. Tamura, S. Shiraishi and Z. I. Takehara, J. Electroanal. Chem., 1995, 394, 49–62 CrossRef.
- A. Lewandowski and A. Świderska-Mocek, J. Power Sources, 2009, 194, 601–609 CrossRef CAS.
- Z. I. Takehara, J. Power Sources, 1997, 68, 82–86 CrossRef CAS.
- Y. Takei, K. Takeno, H. Morimoto and S. I. Tobishima, J. Power Sources, 2013, 228, 32–38 CrossRef CAS.
- P. Verma, P. Maire and P. Novák, Electrochim. Acta, 2010, 55, 6332–6341 CrossRef CAS.
- J. I. Yamaki, I. Yamazaki, M. Egashira and S. Okada, J. Power Sources, 2001, 102, 288–293 CrossRef CAS.
- Y. Y. Lu, M. Tikekar, R. Mohanty, K. Hendrickson, L. Ma and L. A. Archer, Adv. Energy Mater., 2015, 5, 1402073 Search PubMed.
- Y. Y. Lu, Z. Y. Tu and L. A. Archer, Nat. Mater., 2014, 13, 961–969 CrossRef CAS PubMed.
- Y. Y. Lu, Z. Y. Tu, J. Shu and L. A. Archer, J. Power Sources, 2015, 279, 413–418 CrossRef CAS.
- A. A. Arie and J. K. Lee, Diamond Relat. Mater., 2011, 20, 403–408 CrossRef CAS.
- A. A. Arie, O. M. Vovk, J. O. Song, B. W. Cho and J. K. Lee, J. Electroceram., 2008, 23, 248–253 CrossRef.
- M. F. Wu, Z. Y. Wen, Y. Liu, X. Y. Wang and L. Z. Huang, J. Power Sources, 2011, 196, 8091–8097 CrossRef CAS.
- N. J. Dudney, J. Power Sources, 2000, 89, 176–179 CrossRef CAS.
- G. L. Xia, D. Li, X. W. Chen, Y. B. Tan, Z. W. Tang, Z. P. Guo, H. K. Liu, Z. W. Liu and X. B. Yu, Adv. Mater., 2013, 25, 6238–6244 CrossRef CAS PubMed.
- Y. J. Zhang, W. Wang, H. Tang, W. Q. Bai, X. Ge, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2015, 277, 304–311 CrossRef CAS.
- Y. J. Zhang, X. Y. Liu, W. Q. Bai, H. Tang, S. J. Shi, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2014, 266, 43–50 CrossRef CAS.
- Y. J. Zhang, X. H. Xia, D. H. Wang, X. L. Wang, C. D. Gu and J. P. Tu, RSC Adv., 2016, 6, 11657–11664 RSC.
- N. P. Dasgupta, H. B. R. Lee, S. F. Bent and P. S. Weiss, Chem. Mater., 2016, 28, 1943–1947 CrossRef CAS.
- E. Kazyak, K. N. Wood and N. P. Dasgupta, Chem. Mater., 2015, 27, 6457–6462 CrossRef CAS.
- A. C. Kozen, C. F. Lin, A. J. Pearse, M. A. Schroeder, X. Han, L. Hu, S. B. Lee, G. W. Rubloff and M. Noked, ACS Nano, 2015, 9, 5884–5892 CrossRef CAS PubMed.
- K. J. Harry, D. T. Hallinan, D. Y. Parkinson, A. A. MacDowell and N. P. Balsara, Nat. Mater., 2014, 13, 69–73 CrossRef CAS PubMed.
- D. Aurbach, E. Zinigrad, Y. Cohen and H. Teller, Solid State Ionics, 2002, 148, 405 CrossRef CAS.
- C. M. Chen, J. Q. Huang, Q. Zhang, W. Z. Gong, Q. H. Yang, M. Z. Wang and Y. G. Yang, Carbon, 2012, 50, 659–667 CrossRef CAS.
- D. Lin, Y. Liu, Z. Liang, H. W. Lee, J. Sun, H. Wang, K. Yan, J. Xie and Y. Cui, Nat. Nanotechnol., 2016, 11, 626–632, DOI:10.1038/NNANO.2016.32.
- H. Tang, J. P. Tu, X. Y. Liu, Y. J. Zhang, S. Huang, W. Z. Li, X. L. Wang and C. D. Gu, J. Mater. Chem. A, 2014, 2, 5834–5840 CAS.
- H. Tang, X. H. Xia, Y. J. Zhang, Y. Y. Tong, X. L. Wang, C. D. Gu and J. P. Tu, Electrochim. Acta, 2015, 180, 1068–1074 CrossRef CAS.
- S. I. El-Hout, S. M. El-Sheikh, H. M. A. Hassan, F. A. Harraz, I. A. Ibrahim and E. A. El-Sharkawy, Appl. Catal., A, 2015, 503, 176–185 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- X. Q. Niu, X. L. Wang, D. H. Wang, Y. Li, Y. J. Zhang, Y. D. Zhang, T. Yang, T. Yu and J. P. Tu, J. Mater. Chem. A, 2015, 3, 17106–17112 CAS.
- X. Q. Niu, X. L. Wang, D. Xie, D. H. Wang, Y. D. Zhang, Y. Li, T. Yu and J. P. Tu, ACS Appl. Mater. Interfaces, 2015, 7, 16715–16722 CAS.
- Y. Z. Fu, C. X. Zu and A. Manthiram, J. Am. Chem. Soc., 2013, 135, 18044–18047 CrossRef CAS PubMed.
- W. D. Zhou, Y. C. Yu, H. Chen, F. J. DiSalvo and H. D. Abruña, J. Am. Chem. Soc., 2013, 135, 16736–16743 CrossRef CAS PubMed.
- D. H. Wang, D. Xie, T. Yang, Y. Zhong, X. L. Wang, X. H. Xia, C. D. Gu and J. P. Tu, J. Power Sources, 2016, 313, 233–239 CrossRef CAS.
- G. Ma, Z. Wen, Q. Wang, C. Shen, J. Jin and X. Wu, J. Mater. Chem. A, 2014, 2, 19355–19359 CAS.
- G. Ma, Z. Wen, M. Wu, C. Shen, Q. Wang, J. Jin and X. Wu, Chem. Commun., 2014, 50, 14209–14212 RSC.
- Z. Y. Tu, Y. Kambe, Y. Y. Lu and L. A. Archer, Adv. Energy Mater., 2014, 4, 1300654 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13039a |
| This journal is © The Royal Society of Chemistry 2016 |