Reduction of selective polyaromatic nitrotriptycene via an azoxytriptycene intermediate under ambient conditions using a cobalt/cobalt oxide nanocomposite (CoNC)†
Abstract
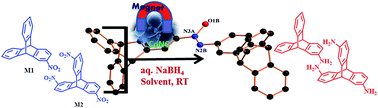
A cobalt-based nanocomposite (CoNC) has been prepared from a recently reported single source molecular precursor (SSMP) [CoII(hep-H)(H2O)4]SO4 (A) (hep-H = 2-(2-hydroxylethyl)pyridine). The resulting nanocomposite material was characterized by using various physicochemical techniques such as XRD, SEM, EDAX, TEM and XPS spectroscopy. X-ray diffraction patterns show the weakly crystalline nature of the catalyst. This was also confirmed by the SAED pattern obtained from HR-TEM. XPS analysis reveals the formation of metallic cobalt and the cobalt oxide (CoO) nanocomposite. CoNC was employed for the facile catalytic hydrogenation of 2-nitrotriptycene (M1) and 2,6,14-trinitrotriptycene (M2) as model substrates under atmospheric reaction conditions, which otherwise takes place either with RANEY® Nickel, Pd/C or SnCl2/HCl catalysts under harsh conditions. The mechanistic pathway reveals that the reduction of M1 proceeds via the intermediacy of azoxy triptycene (III) and N-hydroxylamine triptycene (IV).

 Please wait while we load your content...
Please wait while we load your content...